Anti-tumor vascular drug sustained-release embolization microsphere for malignant tumor interventional therapy
A slow-release embolization and interventional therapy technology, applied in the fields of application, medical science, surgery, etc., can solve the problems of tumor recurrence and metastasis, affect the expected therapeutic effect of TACE, and cannot completely kill tumor cells, so as to inhibit neogenesis and avoid recurrence And the effect of transfer and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
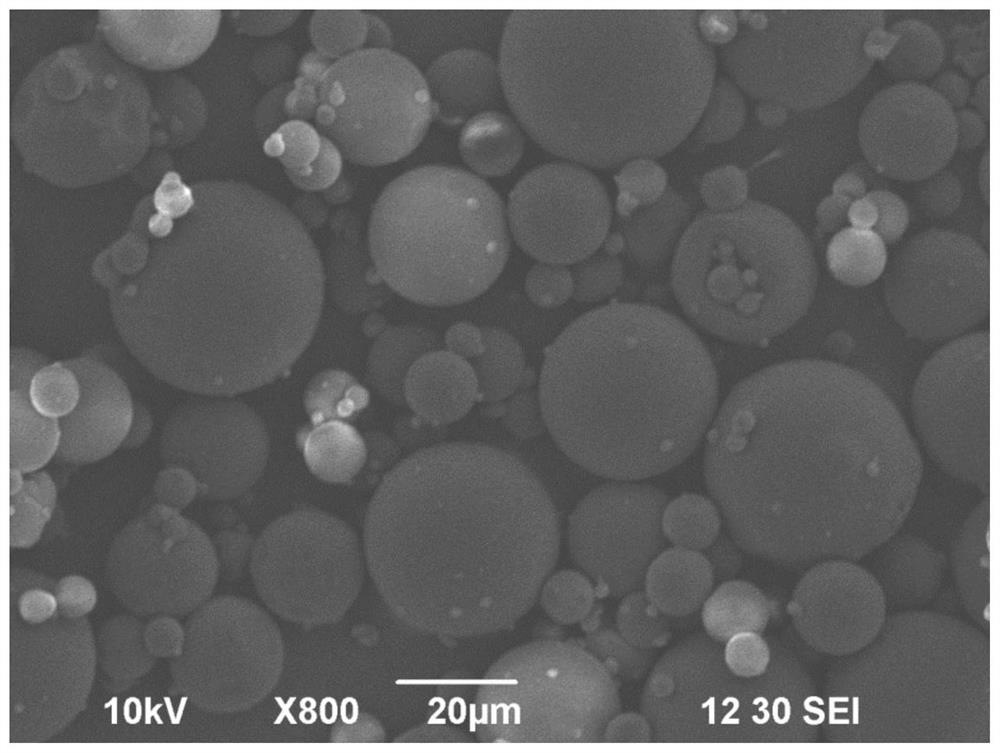
[0054] The anti-tumor vascular drug slow-release embolic microspheres for interventional therapy of malignant tumors has a particle size of 10-1000 μm. It is loaded with anti-tumor vascular drugs for anti-tumor blood vessels, anti-tumor angiogenesis and anti-tumor angiogenesis. The anti-tumor vascular drugs are antibody drugs or small molecular compound drugs, which can resist or inhibit the original blood vessels of tumor tissues and can resist or inhibit the angiogenesis of tumor tissue, thereby inhibiting the formation of collateral circulation of tumor tissue and inhibiting the growth, metastasis and recurrence of tumor, among which
[0055] The above materials are chitin, chitosan or its derivatives, sodium alginate, gelatin, collagen, hyaluronic acid, polypeptide, silk fibroin, starch or its derivatives, polycaprolactone, polylactic acid, polyglycolic acid, Polylactic acid-polyglycolic acid copolymer, polylactic acid polycaprolactone, polylactic acid polycaprolactone cop...
Embodiment 2
[0078] The preparation of embodiment 2 sorafenib tosylate chitosan sustained-release embolism microspheres
[0079] (1) Preparation of microspheres
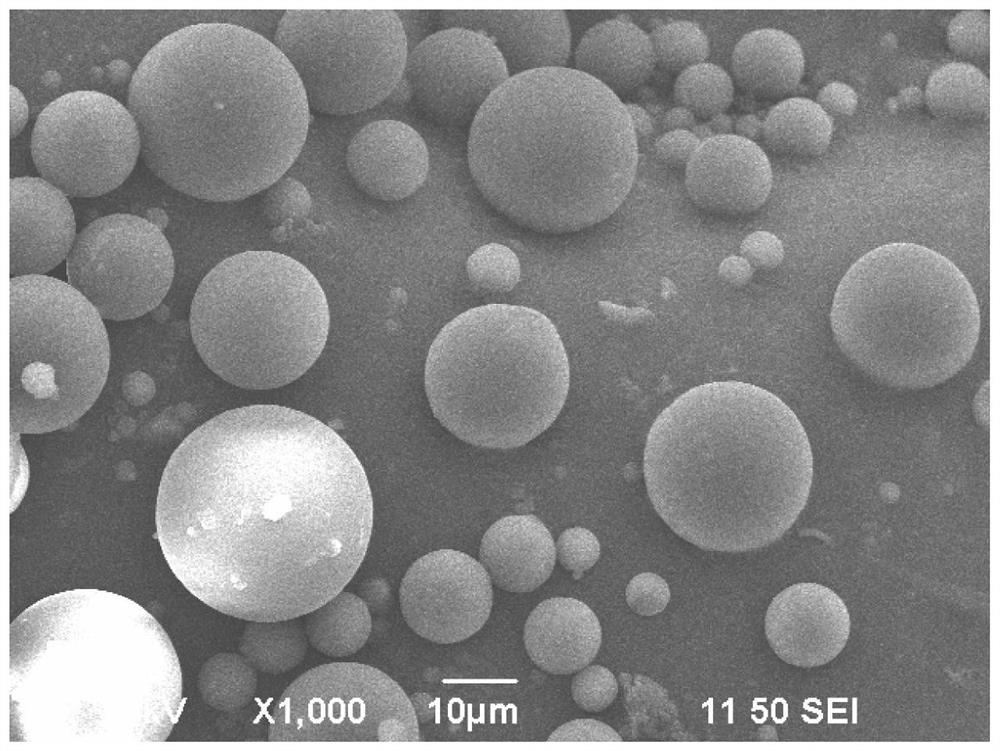
[0080]Dissolve an appropriate amount of bile salt in water, weigh sorafenib tosylate and evenly disperse in the bile salt solution to prepare a drug solution. A proper amount of chitosan was dissolved in 2% acetic acid solution to make 2% chitosan solution. Adding the drug solution into the chitosan solution, adding sodium tripolyphosphate as a cross-linking agent, and mixing evenly to obtain a microsphere preparation solution. Prepared by spray drying method, the equipment used is BUCHI Mini Spray Dryer B-290, the inlet temperature is 130°C, the outlet temperature is 75°C, the flow rate of the preparation solution is 6mL / min, the air pressure is 450kPa, and the air flow rate is 0.7m 3 / min, finally got as figure 1 Sorafenib tosylate chitosan sustained release embolic microspheres shown.
[0081] (2) Encapsulation efficiency...
Embodiment 3
[0093] Example 3 Preparation of Apatinib Mesylate Gelatin Sustained Release Embolization Microspheres
[0094] (1) Preparation of microspheres
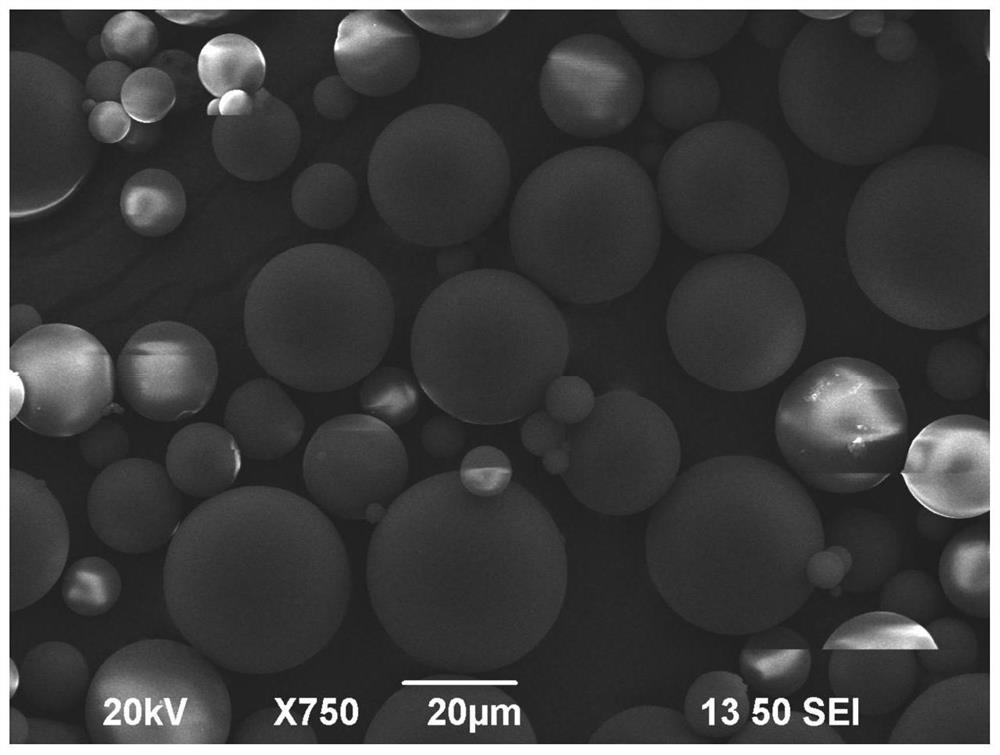
[0095] Apatinib mesylate is dissolved in dilute acetic acid to prepare a drug solution. The prepared gelatin solution with a concentration of 10% is completely dissolved on a water bath at 50-60° C., and then the drug solution is added and mixed evenly to prepare a water phase. Add an appropriate amount of Span-80 to liquid paraffin to form the oil phase, place it in a three-neck flask in a constant temperature water bath at 50°C, and slowly add the water phase to the oil phase drop by drop under the condition of a stirring speed of 200-1000r·min-1 Emulsify in medium, the water-oil ratio is 1:4-1:8. After emulsification to form a stable W / O emulsion, quickly cool down to below 5°C, add formaldehyde or 50% glutaraldehyde to solidify for 1-2 hours, centrifuge the obtained product at 3000r / min, wash 3 times with isopropanol and acetone...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com