Efficient total synthesis method and application of natural product schaftoside
A natural product, the technology of schafposide, used in the fields of chemistry and medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
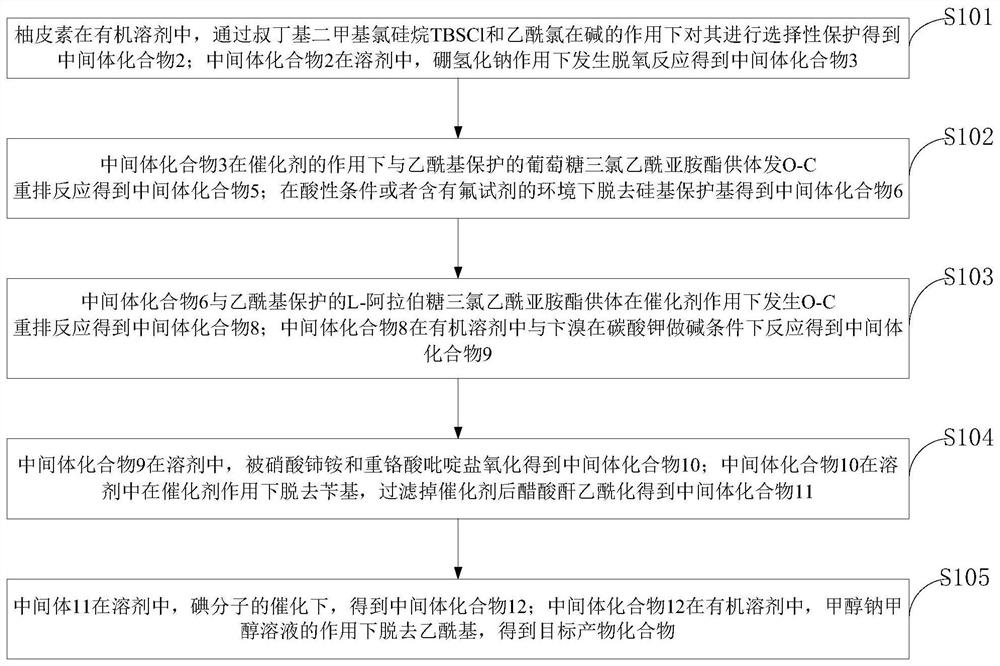
[0043] Such as figure 1 As shown, the efficient total synthesis method of the natural product schaftoside provided by the examples of the present invention comprises the following steps:
[0044] S101, naringenin is selectively protected by tert-butyldimethylsilyl chloride TBSCl and acetyl chloride in an organic solvent to obtain intermediate compound 2; intermediate compound 2 is hydroborated in a solvent A deoxygenation reaction occurs under the action of sodium to obtain intermediate compound 3;
[0045] S102, the intermediate compound 3 undergoes an O-C rearrangement reaction with the acetyl-protected glucose trichloroacetimide ester donor under the action of a catalyst to obtain the intermediate compound 5; the silicon group is removed under acidic conditions or an environment containing a fluorine reagent The protecting group gives intermediate compound 6;
[0046] S103, the intermediate compound 6 and the acetyl-protected L-arabinose trichloroacetimide ester donor und...
Embodiment 1
[0051] The purpose of the present invention is to carry out high-efficiency total synthesis of schafutasides and obtain sufficient amount to expand the application of biological activity.
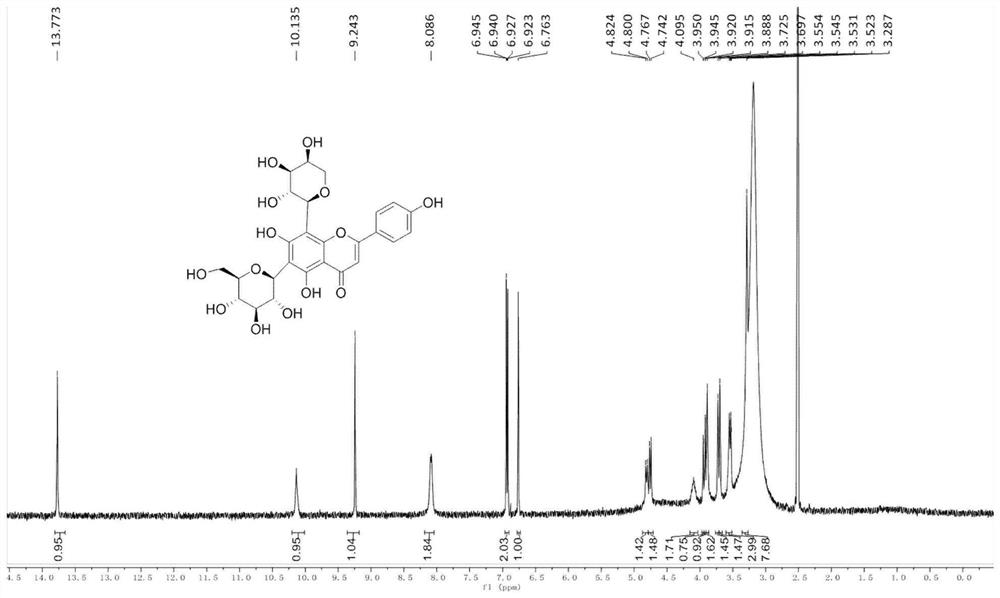
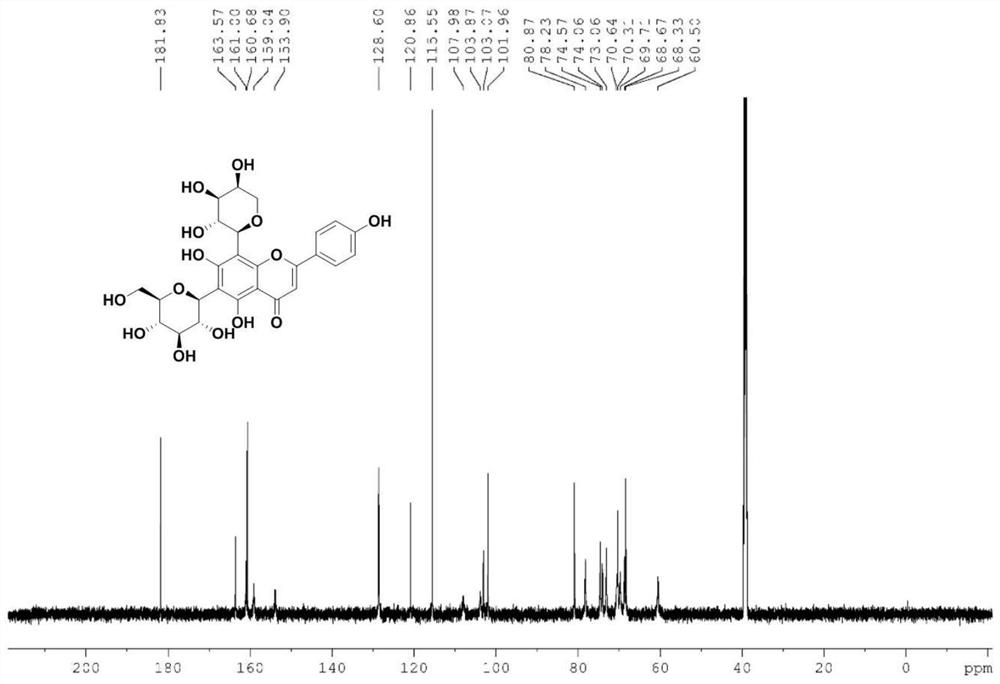
[0052] Related analysis shows that the synthesis of similar flavonoid carbon glycosides is cumbersome and cannot be produced on a large scale. We have established a new method for the synthesis of schafutasides, which has been efficiently synthesized and can be prepared on a large scale. The technical solution of the present invention is, the synthetic method of flavonoid carboglycoside compound schaftoside, and its compound structure is as follows:
[0053]
[0054] The method includes the following steps:
[0055]
[0056] (1) Naringenin was selectively protected by tert-butyldimethylsilyl chloride (TBSCl) and acetyl chloride in an organic solvent to obtain intermediate compound 2. In the reaction, the protecting group at the C7 position of naringenin can be selected from tert-but...
Embodiment 2
[0067] (1) Synthesis of intermediate compound 2:
[0068] Naringenin (5.00g, 18.37mmol), Et 3 N (3.06mL, 22.04mmol) was dissolved in THF (50mL) to lower the reaction temperature to 0°C, then TBSCl (3.32g, 22.04mmol) was dissolved in THF (10mL), and slowly added dropwise to the reaction solution. After 2 hours of reaction at room temperature, TLC (Petroleum–EtOAc, 1:1) monitored the reaction of the raw materials. After the reaction was completed, the temperature of the reaction solution was lowered to 0°C, and Et 3 N (7.66mL, 55.11mmol), then AcCl (3.27mL) dissolved in 20mL THF solution was slowly added dropwise into the solution, paying attention to controlling the internal temperature, and quenching the reaction with saturated aqueous sodium bicarbonate solution after 2h. Dichloromethane ( 3×30 mL) was extracted from the reaction solution, dried over anhydrous sodium sulfate, filtered, and the residue concentrated under reduced pressure was separated by column chromatography...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com