A kind of recombinant bacteria expressing glp-1 analog and its application
A technology of GLP-1 and recombinant bacteria, which is applied in the biological field, can solve the problems of long-term dissolution of inclusion bodies, large renaturation volume, slow growth of protease-deficient strains, and unsuitability for large-scale production, so as to reduce the production of inclusion bodies, Effect of increasing solubility and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
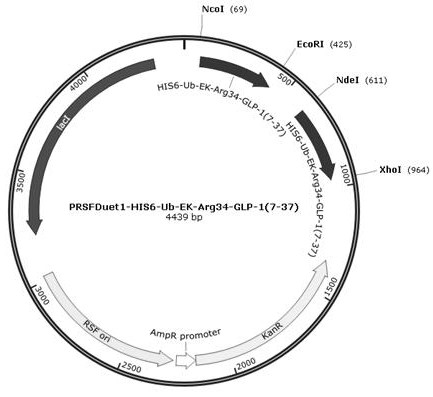
[0050] Example 1 Construction of GLP-1 analog expression vector and protease-inhibiting sRNA vector
[0051] Recombinant fusion gene HIS6-Ub-EK-Arg 34 GLP-1 (7-37) 、HIS6-Ub-EK-Arg 34 GLP-1 (9-37) After double digestion with NcoI and EcoRI, they were respectively connected with the vector fragment recovered from PRSFDuet1 after double digestion with NcoI and EcoRI, and the recombinant expression plasmid PRSFDuet1-HIS6-Ub-EK-Arg was obtained by screening 34 GLP-1 (7-37) , PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (9-37) , and then the recombinant fusion gene HIS6-Ub-EK-Arg 34 GLP-1 (7-37) 、HIS6-Ub-EK-Arg 34 GLP-1 (9-37) After double digestion with NdeI and XhoI, respectively with the plasmid PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (7-37) , PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (9-37) The vector fragments recovered after NdeI and XhoI double digestion were ligated, and the double expression plasmid PRSFDuet1-HIS6-Ub-EK-Arg was obtained by screening 34 GLP-1 (7-37) , PRSFDuet1-HIS6-U...
Embodiment 2
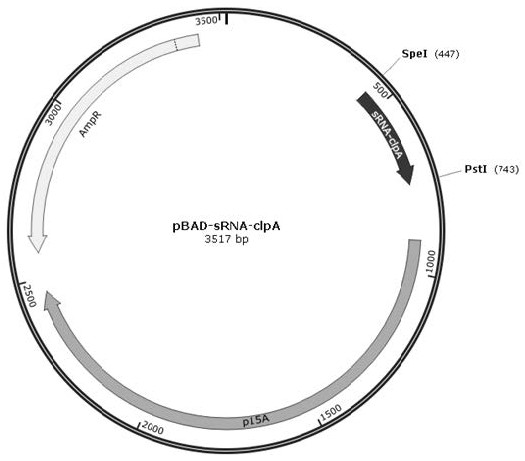
[0053] Example 2 Construction and High Expression Screening of Recombinant Engineering Bacteria Expressing GLP-1 Analogs
[0054] Using BL21 (DE3) as the host bacteria, prepare competent cells, and express recombinant plasmid PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (7-37) , PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (9-37) and plasmid pBAD-sRNA- clpA , pBAD-sRNA- cLP , pBAD-sRNA- QUR , pBAD-sRNA- wxya , pBAD-sRNA- wxya , pBAD-sRNA- Dcp , pBAD-sRNA- PepD and pBAD-sRNA- wxya Transformed into its competent cells by calcium chloride method to form BL21 (DE3) double plasmid expression strain 1 (PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (7-37) and pBAD-sRNA- clpA ), 2 (PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (7-37) and pBAD-sRNA- QUR ), 3 (PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (7-37) and pBAD-sRNA- cLP ), 4 (PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (7-37) and pBAD-sRNA- wxya ), 5 (PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (7-37) and pBAD-sRNA- wxya ), 6 (PRSFDuet1-HIS6-Ub-EK-Arg 34 GLP-1 (7-37...
Embodiment 3
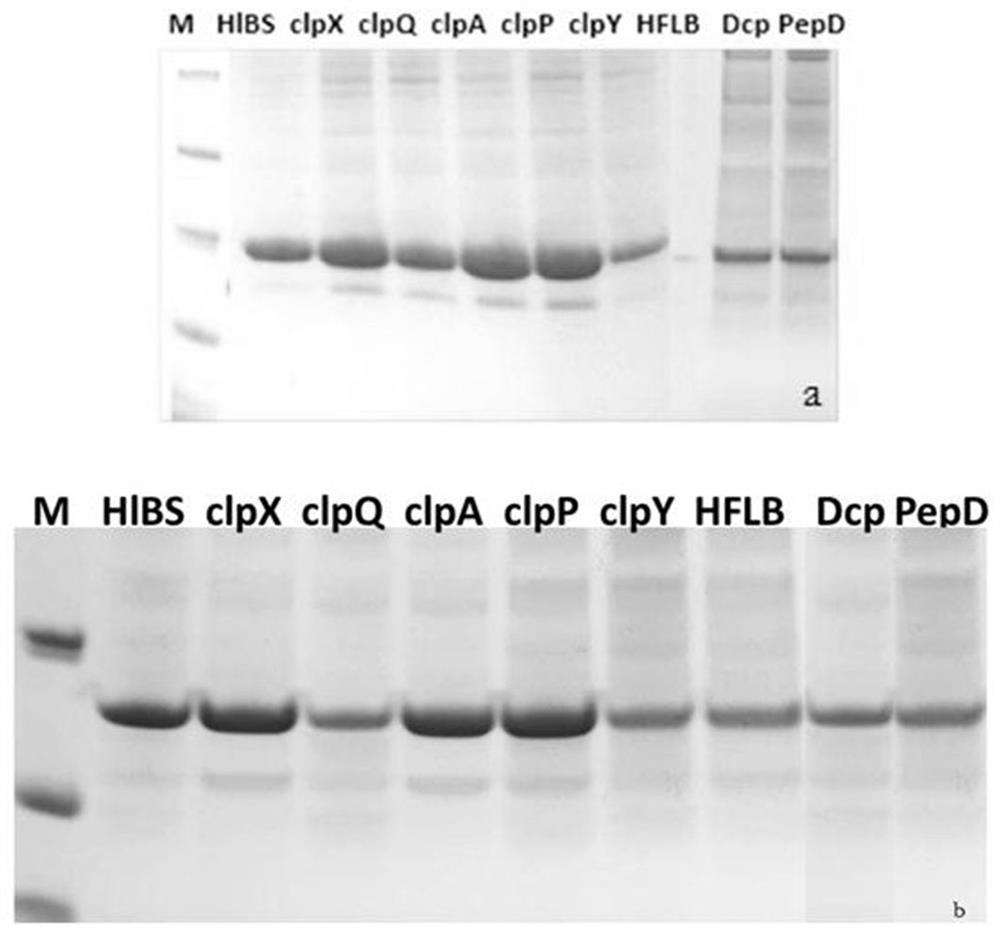
[0055] Example 3 Expression and Purification of Recombinant GLP-1 Analogs
[0056] Pick single colonies of recombinant dual-plasmid expression strain 3 and dual-plasmid expression strain 11 on the plate, and transfer them to a 500mL Erlenmeyer flask at a ratio of 1:1000 for shaking culture at 37°C after 8 hours of test tube culture. When the OD value reaches 0.6-0.8, use 0.5 mM IPTG was used for induction, and after induction at 25°C for 20 h, the supernatant was centrifuged to obtain bacterial pellets.
[0057] Suspend the cells obtained by centrifugation with 30 mL of protein-purified Buffer A, and use a high-pressure homogenizer to crush the suspended cells to obtain a crude protein sample solution, collect the supernatant by centrifugation, and use Affinity Ni to purify the protein. Different proportions of Buffer B were used for gradient elution to elute the protein containing HIS6, and the eluate was desalted and replaced into the solution Buffer C. Fusion protein isola...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com