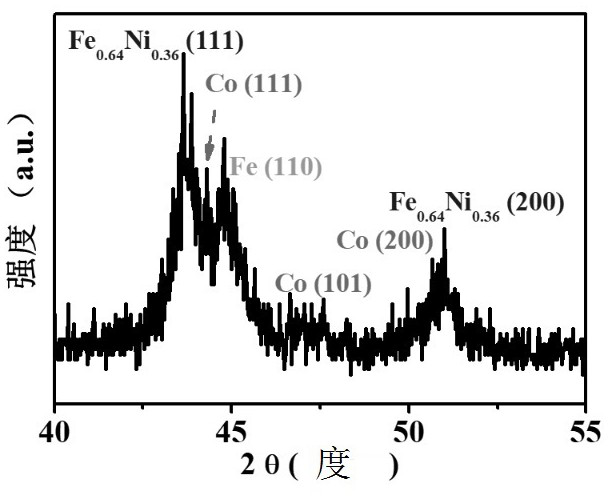
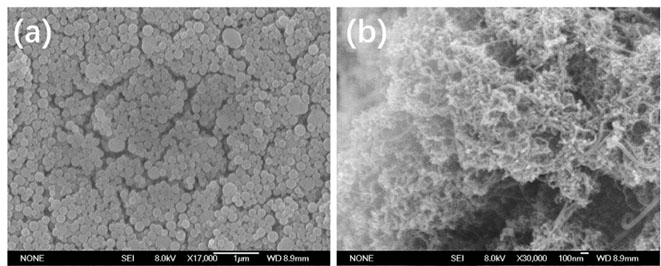
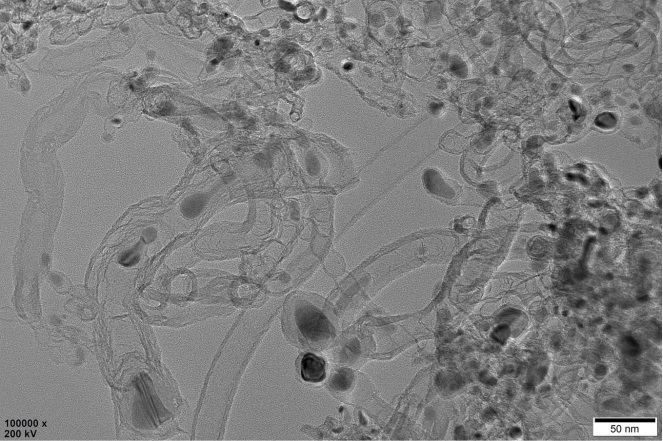
Preparation method of oxygen reduction and oxygen evolution bifunctional catalyst
A dual-functional catalyst and oxygen evolution technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems such as inconvenience, metals reduce the high catalytic activity of nanomaterials, and limit catalyst performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The invention provides a method for preparing an oxygen reduction and oxygen evolution dual-functional catalyst, comprising the following preparation steps:
[0021] Step 1 Synthesis of spherical Prussian blue analogs
[0022] Weigh two transition metal salts, dissolve the two transition metal salts in deionized water at the same time to form mixed solution A, then weigh potassium cyanide and structure directing agent and dissolve them in deionized water to prepare solution B. Mix solution A and solution B, stir evenly, and let stand to react. The product is taken out, washed, and freeze-dried to obtain a dry precursor, which is a spherical Prussian blue analogue.
[0023] Metals in transition metal salts include Cu, Ni, Fe, Co, Cr, Zn, Ag; salts in transition metal salts include nitrates, acetates, chlorides or sulfates. For example: nitrate, acetate, chloride or sulfate of Cu, nitrate, acetate, chloride or sulfate of Ni, nitrate, acetate, chloride or sulfate of Fe, ...
Embodiment 1
[0037](1) Weigh 0.2 g of cobalt acetate tetrahydrate and 0.1 g of nickel acetate tetrahydrate and dissolve them in 100 mL of deionized water as solution A.
[0038] (2) Weigh 0.2 g of potassium ferricyanide and 3.5 g of sodium lauryl sulfate and dissolve them in 100 mL of deionized water as solution B.
[0039] (3) Solution A and solution B were mixed and kept stirring for 15 min, then left to stand at room temperature for 24 h, and then the solution and the precipitate were centrifuged and washed three times with deionized water to obtain the Prussian blue analog powder.
[0040] (4) The powder was mixed with 8 g of urea and placed in an argon-protected tube furnace. The temperature was raised to 600° C., kept for a period of time, and then naturally cooled to room temperature to obtain a carbon nanotube catalyst.
Embodiment 2
[0042] (1) Weigh 0.4 g of ferric acetate hydrate and 0.2 g of nickel acetate tetrahydrate and dissolve them in 100 mL of deionized water as solution A.
[0043] (2) Dissolve 0.2 g potassium cobaltcyanide and 3.0 g polyvinylpyrrolidone in 100 mL deionized water as solution B.
[0044] (3) Solution A and solution B were mixed and kept stirring for 15 min, then left to stand at room temperature for 24 h, and then the solution and the precipitate were centrifuged and washed three times with deionized water to obtain the Prussian blue analog powder.
[0045] (4) The powder was mixed with 8 g of melamine and placed in an argon-protected tube furnace, the temperature was raised to 800 ° C, and after a period of heat preservation, it was naturally cooled to room temperature to obtain a carbon nanotube catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com