Preparation method of co1-xs-mos2-nitrogen-doped carbon her/oer bifunctional catalyst
A bifunctional catalyst, nitrogen-doped carbon technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem of underreporting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
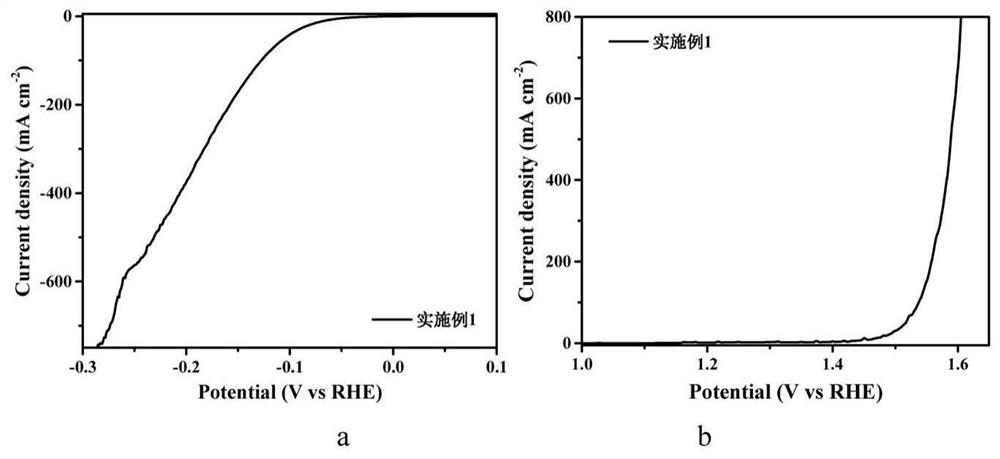
[0033] CoCl 2 ·6H 2 O and urea were dissolved in 40 mL of deionized water at room temperature, in which CoCl 2 The concentration of urea is 0.15M, and the mass fraction of urea is 6.25wt.%. Take the hydrophilic carbon paper and immerse it in the solution, and then keep it at 90°C for 2 hours. After cooling to room temperature, take out the carbon paper and rinse it with deionized water three times. , dry for later use. Then pre-oxidize in the air at 400°C for 0.5h, and then sinter at 600°C in S atmosphere for 2h to obtain Co 9 S 8 array of nanorods. Dissolve 0.492g of molybdenum chloride and 100mg of bipyridine in 2.85ml of N,N dimethylformamide and 0.15ml of Tx-100 solution, stir and dissolve to obtain a 600mM molybdenum chloride nitrogen carbon solution. The above grown Co 9 S 8 The carbon paper of the nanorod array was soaked in the molybdenum chloride nitrogen carbon solution at room temperature for 1 min, and then dried on a hot stage at 80°C for 10 min to obtain C...
Embodiment 2
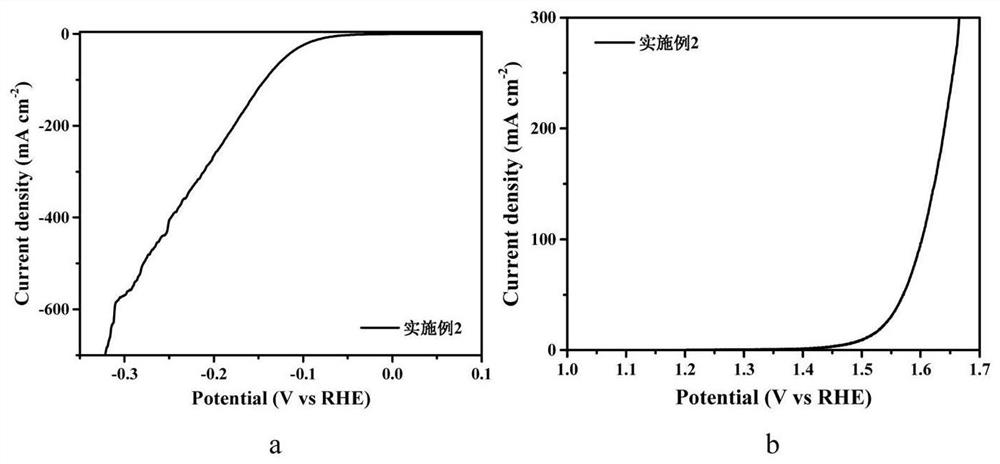
[0048] The preparation steps are the same as in Example 1, only the quality of molybdenum chloride is 0.164g, and the molybdenum chloride nitrogen carbon solution of 300mM is obtained, and Co 1-x S-MoS 2 - Nitrogen doped carbon (NC) in situ electrodes.
[0049] figure 2 Electrode prepared for Example 2 (a) HER linear voltammetry (LSV) diagram and (b) OER LSV diagram. It can be seen from figure (a) that when the current density of the electrode is 10mA / cm 2 , the overpotential required for HER reaction to produce hydrogen in alkaline aqueous solution is only 80mV; when the current density is 100mA / cm 2 When the corresponding overpotential is only 143mV; when the current density is 400mA / cm 2 , the corresponding overpotential is only 248mV. This reflects the excellent catalytic hydrogen evolution performance of the material. It can be seen from the figure (b) that when the current density of the electrode is 10mA / cm 2 , the overpotential corresponding to OER reaction oxyge...
Embodiment 3
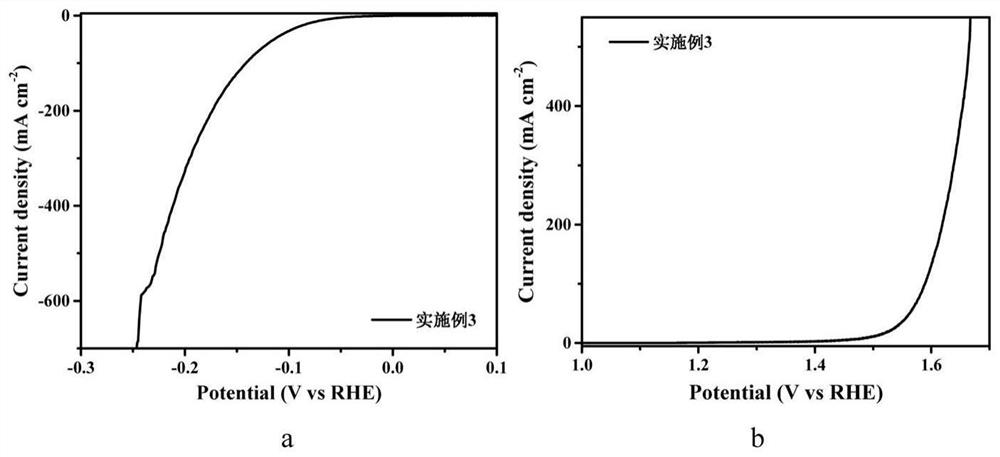
[0051] The preparation steps are the same as in Example 1, only the quality of molybdenum chloride is 0.328g, and the molybdenum chloride nitrogen carbon solution of 400mM is obtained, and Co 1-x S-MoS 2 - Nitrogen doped carbon (NC) in situ electrodes.
[0052] image 3 Electrodes prepared for Example 3 (a) HER linear voltammetry (LSV) diagram and (b) OER LSV diagram. It can be seen from figure (a) that when the current density of the electrode is 10mA / cm 2 , the overpotential required for HER reaction to produce hydrogen in alkaline aqueous solution is only 70mV; when the current density is 100mA / cm 2 When the corresponding overpotential is only 142mV; when the current density is 400mA / cm 2 , the corresponding overpotential is only 212mV. This reflects the excellent catalytic hydrogen evolution performance of the material. It can be seen from the figure (b) that when the current density of the electrode is 10mA / cm 2 , the overpotential corresponding to OER reaction oxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com