Regeneration method and application of chloronitro aromatic hydrocarbon selective hydrogenation catalyst
A technology for hydrogenation catalysts and nitroaromatic hydrocarbons, applied in catalyst regeneration/reactivation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc. Loss, recovery of deactivated catalyst specific surface area and pore volume are limited
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: The 5w% Pt / C catalyst deactivated in the selective hydrogenation of o-chloronitrobenzene to prepare 2,2'-dichlorohydroazobenzene is regenerated, comprising the following steps:
[0034] (1) Take 10g of deactivated catalyst (referred to as Cat1-U) and disperse it into 50g of methanol solution containing 5% n-butyl titanate and 5% terephthalaldehyde, stir and impregnate at 50°C, and the impregnation is completed Afterwards, the catalyst was filtered and transferred to a tube atmosphere furnace under N 2 Under the protection of 2°C / min, the heating rate was increased to 500°C and then maintained for 2h for carbonization treatment. The product obtained after carbonization (denoted as N&Ti@Cat1-U) was cooled and stored for later use.
[0035] (2) Add 10g of N&Ti@Cat1-U and 250g of Cat1-U to a 10L regenerating kettle equipped with an ultraviolet light irradiation device and a microwave generating device, add 2.5kg of an aqueous solution containing 5% sodium pero...
Embodiment 2
[0049] Embodiment 2: The 3w% Pd / C catalyst deactivated in the selective hydrogenation of o-chloronitrobenzene to prepare 2,2'-dichlorohydroazobenzene is regenerated, comprising the following steps:
[0050] (1) Take 10g of deactivated catalyst (Cat2-U) and disperse it into 50g of methanol solution containing 15% n-butyl p-titanate and 15% terephthalaldehyde, and stir and impregnate it at 80°C. The catalyst was filtered and transferred to a tube atmosphere furnace under N 2 Under the protection of the temperature rising rate of 5 ℃ / min to 800 ℃ and then maintained for 2 hours for carbonization treatment, the product obtained after carbonization (denoted as N&Ti@Cat2-U) was cooled and stored for later use.
[0051] (2) Add 10g of N&Ti@Cat2-U and 300g of Cat2-U to a 10L regenerating kettle equipped with an ultraviolet light irradiation device and a microwave generating device, and add 3kg of an aqueous solution containing 5% potassium peroxodisulfate. The regeneration treatmen...
Embodiment 3
[0057] Embodiment 3: comparative experiment of regeneration method
[0058] (1) Add 250g of Cat1-U directly to a 10L regeneration kettle equipped with a UV irradiation device and a microwave generator, add 2.5kg of an aqueous solution containing 5% sodium peroxodisulfate, and irradiate it with ultraviolet light with a wavelength of 200nm Regeneration treatment is carried out under the action, the treatment temperature is 50°C, and the microwave power is 1000W. The processed material liquid is filtered by nitrogen pressure into the precision filter.
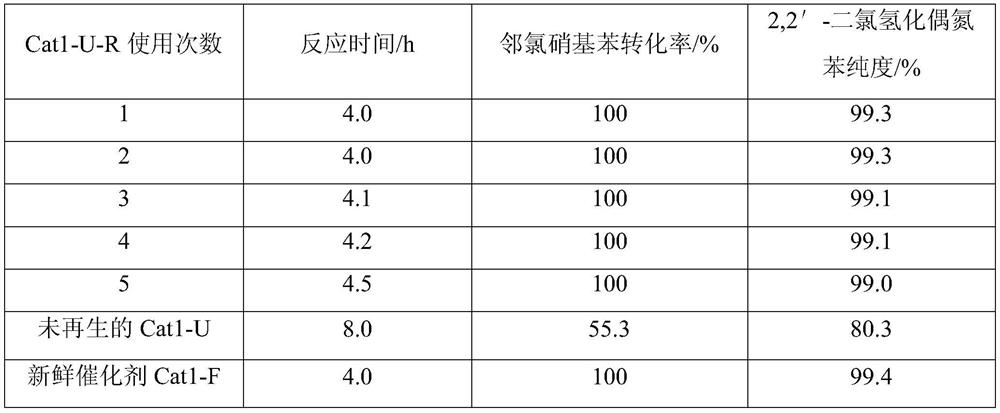
[0059] (2) Carry out subsequent regeneration experiments according to steps (3) and (4) of Example 1. Catalyst performance evaluation is carried out to the regenerated catalyst (referred to as Cat1-U-R1): evaluation method and analysis method are the same as embodiment 1. The result is as follows:
[0060]
[0061] As can be seen from the experimental results in the above table, compared with the catalyst regenerated in Examp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com