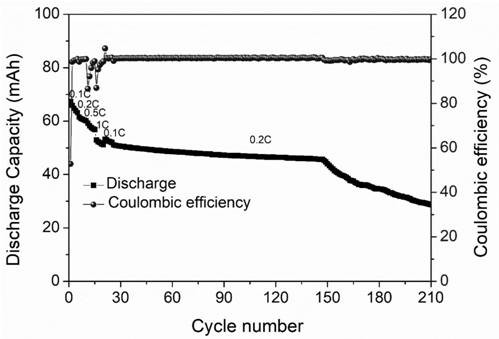
Wide-temperature self-healing electrolyte, preparation method thereof and lithium battery
A technology of electrolyte and lithium battery, which is applied in the field of wide-temperature self-healing electrolyte and its preparation, and can solve problems such as service capability decline, device short circuit, and capacity reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The present invention also provides a method for preparing the wide temperature self-healing electrolyte described in the above technical solution, comprising the following steps:
[0041] S1. N-ethylethanolamine and carbon disulfide are reacted, and the product obtained is recorded as TDS;
[0042] In step S1, the reaction solution is iodine-containing chloroform, and the reaction condition is ice bath. The molar ratio of N-ethylethanolamine and carbon disulfide is 1:(0.5-1).
[0043] S2. reacting diisocyanate with hydroxyl-terminated polyether polyol to obtain isocyanate-terminated polyether polyol;
[0044] In step S2, the diisocyanate is toluene diisocyanate (TDI), isophorone diisocyanate (IPDI), diphenylmethane diisocyanate (MDI), dicyclohexylmethane diisocyanate (HMDI), hexamethylene One or more in radical diisocyanate (HDI), lysine diisocyanate, preferably toluene diisocyanate; Described polyether polyol is one or more in polyethylene glycol, polypropylene glycol...
Embodiment 1
[0051] A wide temperature self-healing electrolyte, the preparation method comprising:
[0052] S1. Add 17.8g of N-ethylethanolamine and 100ml of chloroform into the flask and mix and stir in an ice bath, then add 6ml of carbon disulfide and 12.7g of iodine to react for 3 hours, then remove impurities, and record the product as TDS.
[0053] S2. Reaction of 2,4-toluene diisocyanate and hydroxyl-terminated polypropylene glycol in chloroform at 80°C to obtain 2,4-toluene diisocyanate-terminated polypropylene glycol (molecular weight is about 2300Da)
[0054] S3. Dissolve 2 mmol of glycerol in 50 mL of chloroform CHCl 3 and pour it into a 100ml three-necked flask, then add 0.1mmol of dibutyltin dilaurate (DBTDL) into the flask, then add 6mmol of 2,4-toluene diisocyanate-terminated polypropylene glycol and 1mmol of TDS, and react at 60°C for 5h ; Pour into a polytetrafluoroethylene mold to volatilize to form a film, and obtain a wide temperature self-healing electrolyte membrane....
Embodiment 2-3 and comparative example 1
[0059] A wide temperature self-healing electrolyte, compared with Example 1, the difference is that the molar ratio of glycerol, TDS and 2,4-toluene diisocyanate-terminated polypropylene glycol is shown in Table 1, and the others are roughly the same as Example 1, I won't repeat them here.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Breaking strength | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com