Voriconazole synthesis process
A synthetic process, voriconazole technology, applied in the field of drug synthesis, can solve the problems of low product purity and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
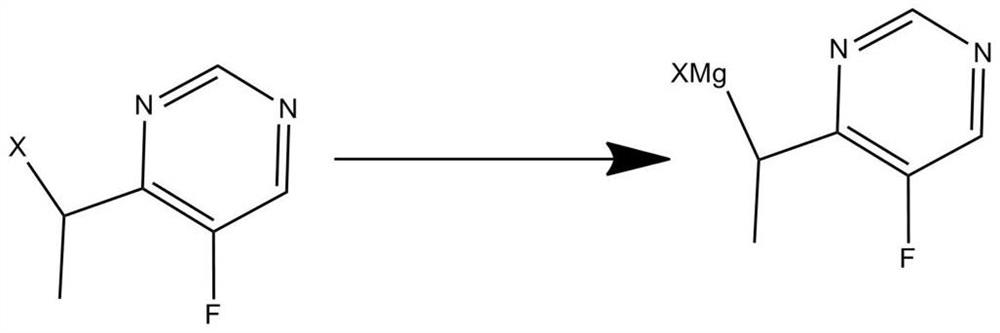
[0075] In a flask equipped with a double-necked adapter, place 4.8 g of magnesium chips. The main mouth of the interface is equipped with a dropping funnel, and the side mouth is equipped with a reflux condenser of the calcium chloride drying tube. Add dropwise a mixed solution of 0.2mol 4-(1-bromoethyl)-5-fluoropyrimidine and 302mL methyltetrahydrofuran into the bottle, and heat in a water bath at a temperature of 50°C to obtain a solution of Grignard reagent in methyltetrahydrofuran;
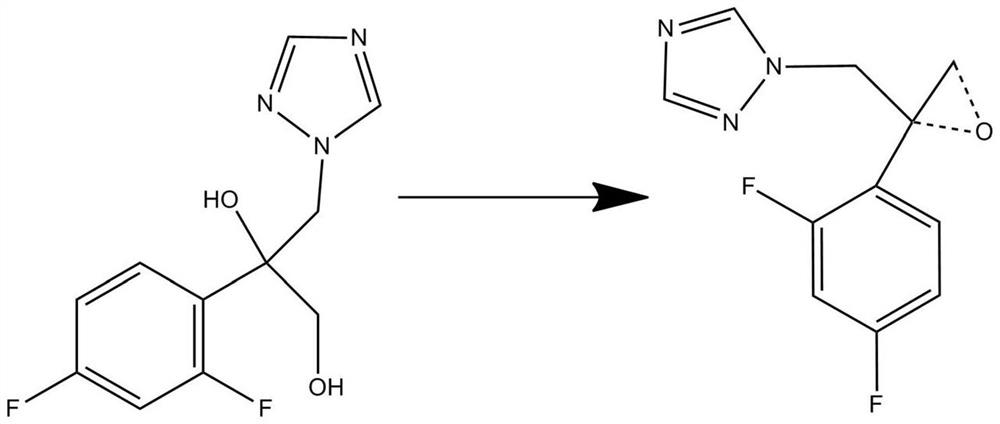
[0076] Weigh 0.2mol 2-(2,4-difluorophenyl)-3-(1,2,4-triazol-1-yl)-1,2-propanediol and 0.05mol potassium oxide-silica supported catalyst (wherein the loading of potassium oxide is 12wt%), the reaction temperature is 65°C, and the reaction pressure is 2MPa; 2-(2,4-difluorophenyl)-3-(1,2,4-triazole- 1-yl)-1,2-propylene oxide;
[0077] Take 0.1mol of 2-(2,4-difluorophenyl)-3-(1,2,4-triazol-1-yl)-1,2-epoxypropane dissolved in 1.2mol of carbon dioxide Add 100 mL of the prepared Grignard reagent i...
Embodiment 2
[0079] 0.05 mol of the voriconazole racemate prepared in Example 1 was dissolved in 73.7 mL of acetone, and 13.9 g of L-(-)-10-camphorsulfonic acid dissolved in 24 mL of methanol was added thereto. The resulting mixture was refluxed for 1 h, and slowly cooled to room temperature, crystallized, filtered, and dried to obtain the camphorsulfonate of voriconazole;
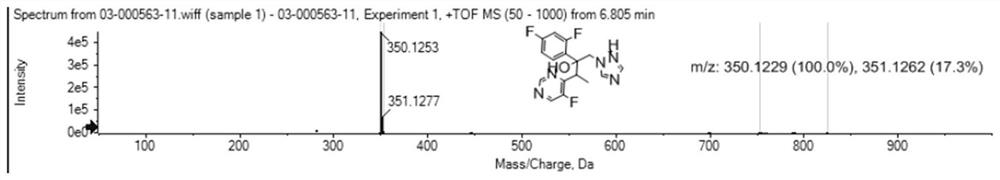
[0080] Voriconazole camphorsulfonate was added to 100 mL of water and dichloromethane at a volume ratio of 1:1, and 40% sodium hydroxide solution was slowly added thereto to adjust the pH to 11-12. The organic layer was separated, dried, and the organic solvent was removed under reduced pressure, crystallized from isopropanol, and dried to obtain 13.7 g of white voriconazole, with a yield of 92%, and the mass spectrum of the obtained voriconazole was as follows: figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com