Method for photocatalytic dissolution of metal by using phosphate radical modified photocatalyst
A photocatalyst and phosphate radical technology, which is applied in chemical instruments and methods, physical/chemical process catalysts, and improvement of process efficiency. Catalyst activity etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A method for photocatalytically dissolving metals using a phosphate-modified photocatalyst, comprising the following steps:
[0038] (1) Preparation of inorganic phosphate-modified photocatalysts
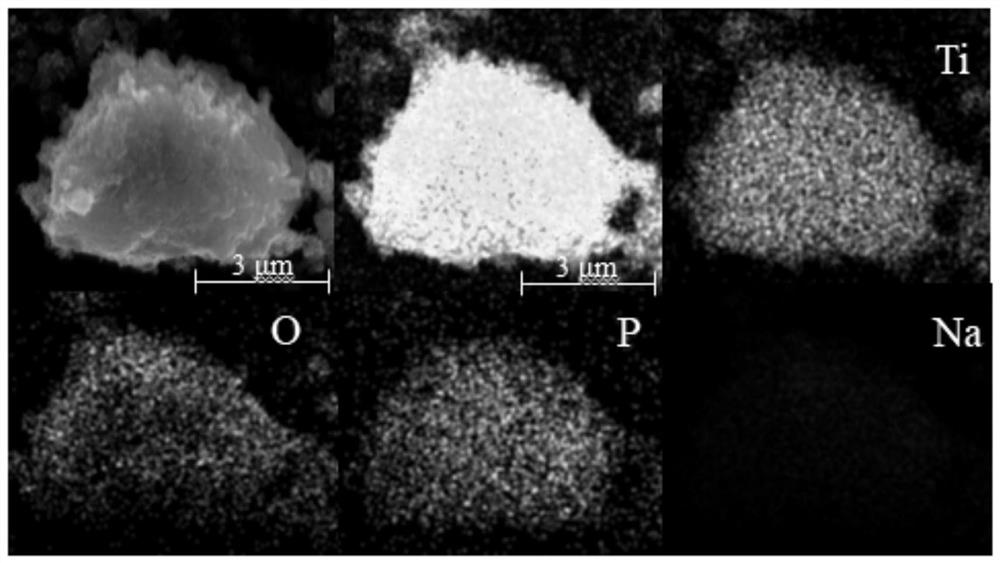
[0039] Take 0.1g K 3 PO 4 Add it into 100mL deionized aqueous solution, stir at room temperature for 1h, add 0.1g of titanium dioxide during stirring, and continue stirring at 90°C for 20h. After centrifugal separation and drying, the phosphate-modified photocatalyst was obtained. attached figure 1is the SEM image of the obtained phosphate-modified titanium dioxide, as can be seen from the element distribution diagram, uniform stirring for a certain period of time makes the phosphate evenly distributed on the surface of the titanium dioxide photocatalyst, and obtains an inorganic phosphate-modified photocatalyst, its structural formula as follows.
[0040]
[0041] (2) Dissolved metal
[0042] Disperse 0.1 g of the metal-containing material to be dissolved (Au ore cr...
Embodiment 2
[0050] A method for photocatalytically dissolving metals using a phosphate-modified photocatalyst, comprising the following steps:
[0051] (1) Preparation of inorganic phosphate-modified photocatalysts
[0052] Take 1g, 2g, 3g, 4g, 5g K respectively 3 PO 4 Add 100 mL of deionized water solution, stir at room temperature for 1 h, add 0.1 g of titanium dioxide during stirring, and continue stirring at 90° C. for 20 h. After centrifugal separation and drying, the phosphate-modified photocatalyst was obtained. attached figure 1 It is the EDS mapping diagram of the obtained phosphate-modified titanium dioxide. It can be seen from the element distribution diagram that the phosphate is evenly distributed on the surface of the titanium dioxide photocatalyst, and the mass percentages of the phosphate are 1%, 2%, and 3%, 4%, 5% photocatalyst.
[0053] The object of photocatalytic dissolution of metal is silver, that is, extract the metal Ag in the Ag ore, crush the Ag ore to 10-20...
Embodiment 3
[0059] Repeat the operation steps of Example 2, the difference is that the object of photocatalytic dissolution of metal is platinum, that is, the metal Pt in the Pt ore is extracted, and the Pt ore is pulverized to 10-20nm, the results are shown in Table 3 below
[0060] The dissolution rate and dissolution rate of Pt were detected by inductively coupled plasma spectrometer (ICP).
[0061] The results obtained are shown in Table 3 below:
[0062]
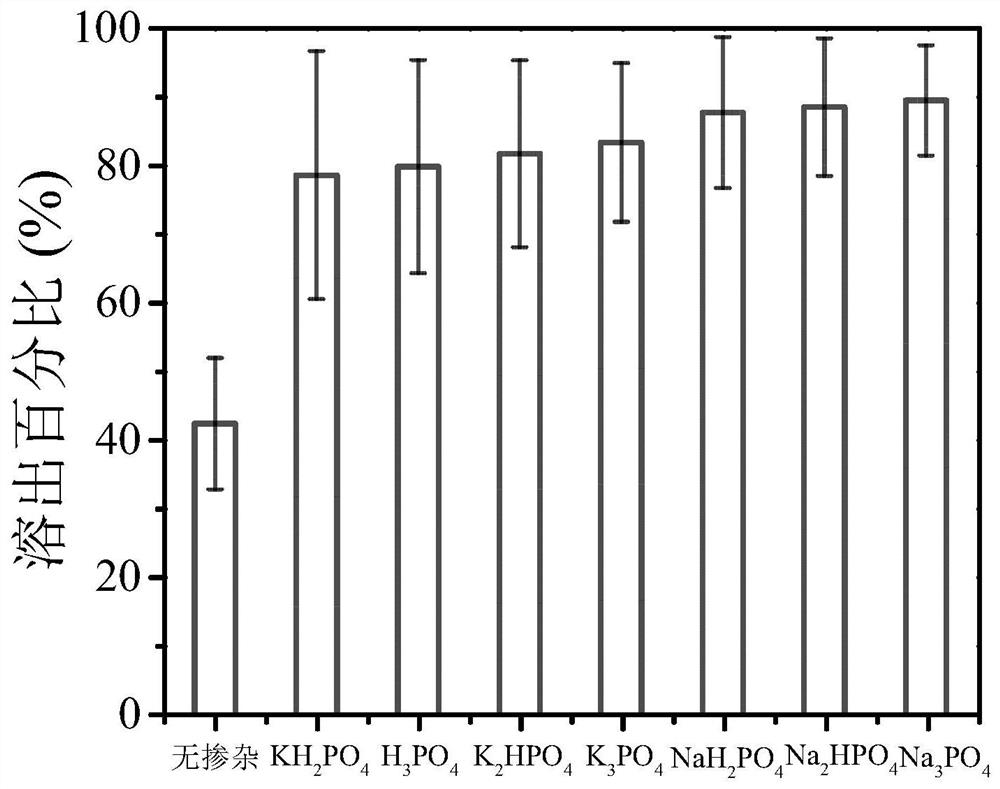
[0063] It can be seen from the above table that the rate of photocatalytic dissolution of platinum can be significantly improved with the increase of the modification amount of phosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com