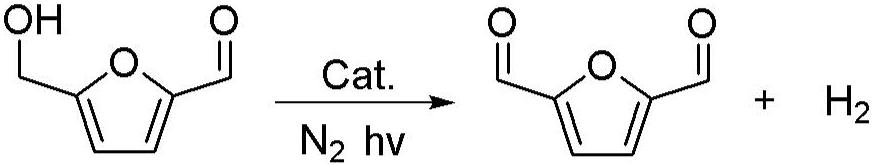
Method for preparing 2,5-furandicarboxaldehyde through photocatalytic dehydrogenation of 5-hydroxymethylfurfural
A technology for hydroxymethyl furfural and furandiformaldehyde, which is applied in the field of photocatalytic 5-hydroxymethyl furfural dehydrogenation and preparation 2, can solve problems such as explosiveness, side reactions, difficult separation of target products and by-products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
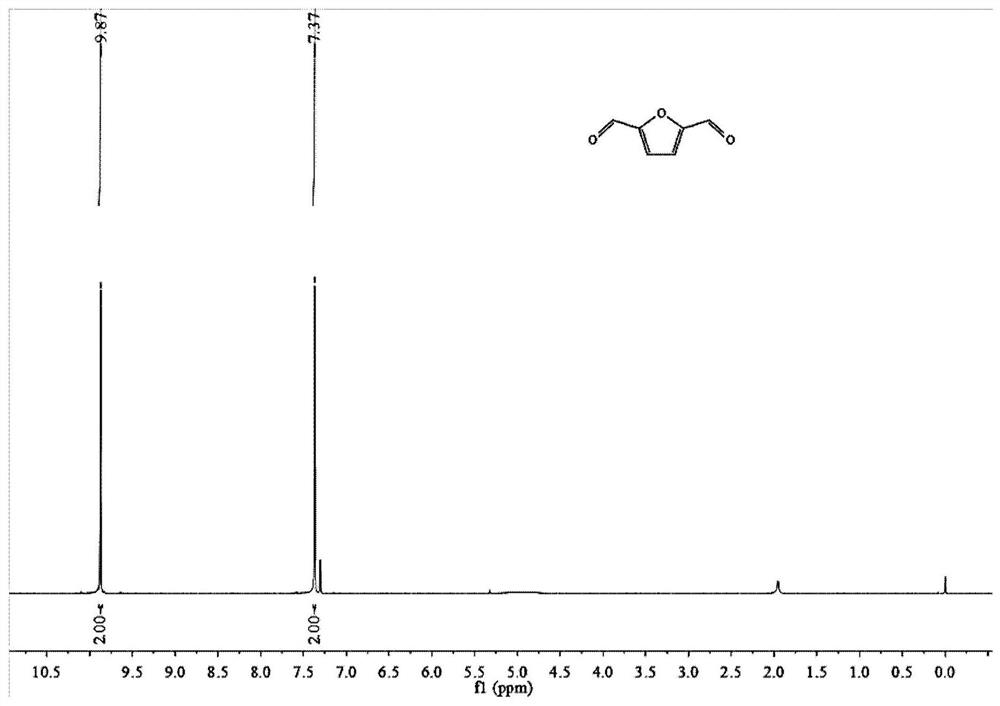
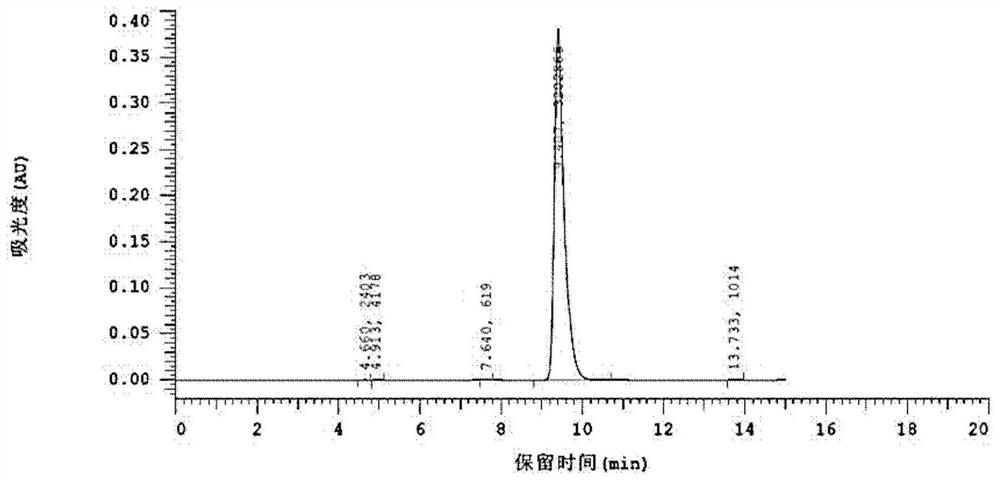
[0030] Put 0.1g of 5-hydroxymethylfurfural (Chinese medicine) into a 25ml schlenk tube after crushing, add 10mg of Rh / C 3 N 4 , 1ml of acetonitrile (Chinese medicine), the residual air in the nitrogen replacement reactor is irradiated with light with a wavelength of 390nm in the light reactor, the temperature is adjusted so that the temperature reaches 25 ° C, the reaction is kept for 12 hours, and the stirring rate is 500r / min. After the reaction, the reaction solution was transferred to a sample bottle, and the sample was centrifuged and then sent to a high-performance liquid chromatograph. Liquid phase conditions: chromatographic column: Zorbax-ODS column, 4 × 150mm; column temperature: 30 ° C; mobile phase: methanol: Water=20:80; flow rate 0.6mL / min; injection volume: 20uL. The resulting product is 2,5-furandicarbaldehyde (see the hydrogen spectrum figure 1 , see liquid phase figure 2 ), and the yield was 96.8%.
Embodiment 2
[0032] The specific preparation process and detection method are the same as in Example 1, the difference is only that the Rh / C 3 N 4 change to C 3 N 4 . The product obtained was 2,5-furandicarbaldehyde, and the yield was 92%.
Embodiment 3
[0034] The specific preparation process and detection method are the same as in Example 1, the difference is only that the Rh / C 3 N 4 Change to Pt / C 3 N 4 . The obtained product was 2,5-furandicarbaldehyde, and the yield was 94.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com