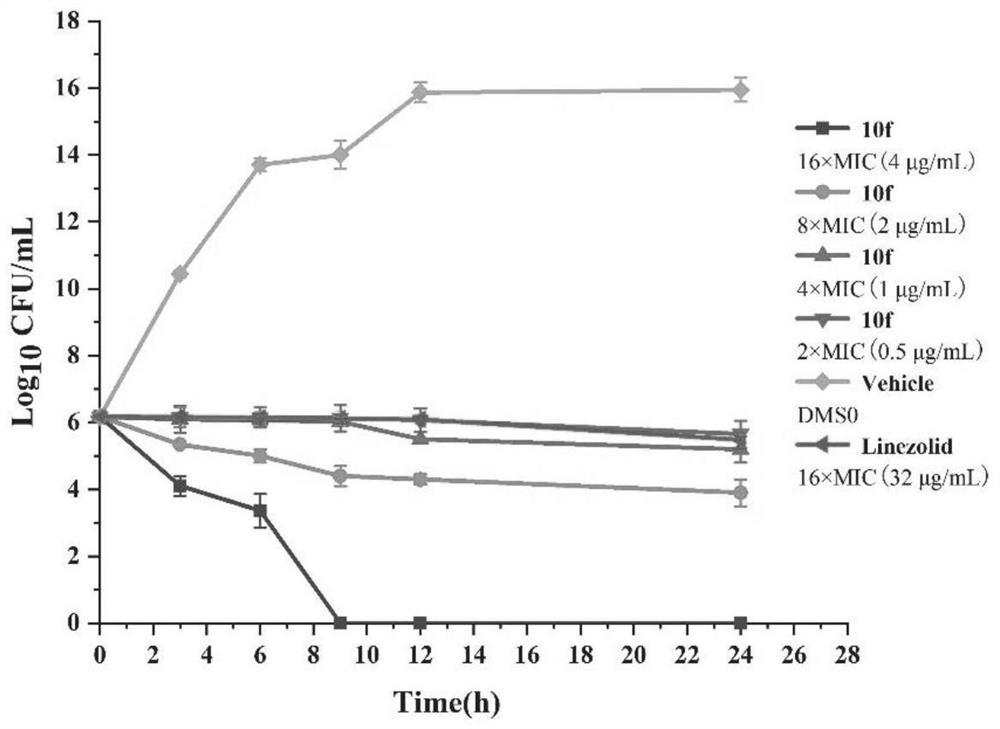
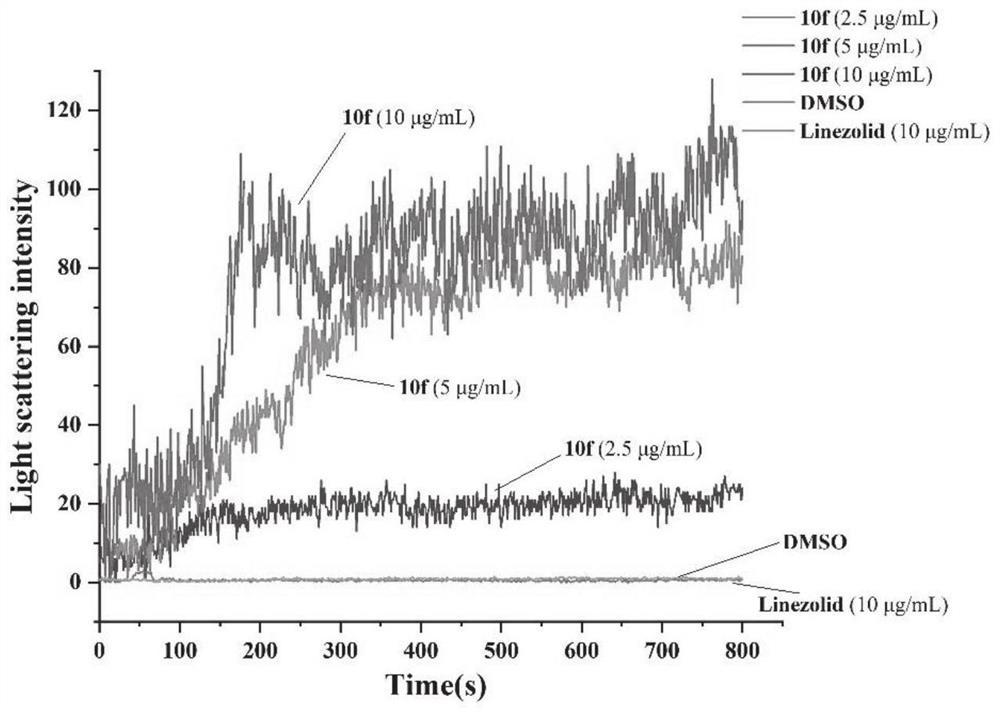
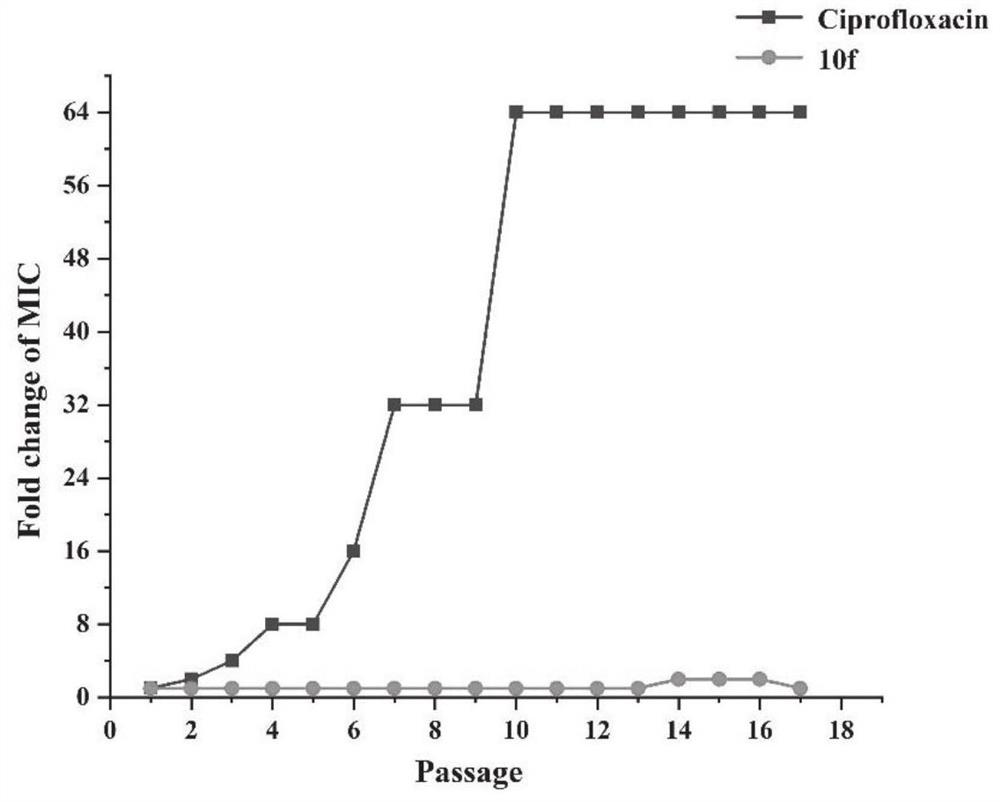
Aromatic heterocycle substituted acridine quaternary ammonium salt derivative as well as preparation method and application thereof
A technology of aromatic heterocycles and quaternary ammonium salts, applied in the field of pharmaceutical compounds, can solve the problems of high side effects and antibacterial activity to be further improved, and achieve good bactericidal effect and significant interference effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of the acridine quaternary ammonium salt derivatives substituted by the aromatic heterocycle comprises the following reaction scheme:
[0044]
[0045] Among them, R 1 , X are all as above.
[0046] Specifically, the preparation method: first carry out Ullmann reaction to obtain followed by intramolecular cyclization to obtain followed by a nucleophilic substitution reaction to obtain followed by a nucleophilic substitution reaction to obtain Finally, the compound shown in formula (I) is obtained through nucleophilic substitution and rearrangement.
[0047] with R 1 for As an example, the preparation route looks like this:
[0048]
[0049] Operation steps: o-bromobenzoic acid and aniline undergo Ullman condensation, intramolecular ring closure, nucleophilic substitution reaction with methyl iodide, and then chlorination to obtain intermediate 5. The aromatic heterocyclic substituent is prepared by nucleophilic substitution, and...
Embodiment 1
[0068] Preparation of 2-(phenylamino)benzoic acid (2)
[0069]
[0070] Weigh raw material 2-bromobenzoic acid (2.0g, 10mmol), aniline (1.85g, 20mmol), potassium carbonate (1.37g, 10mmol) and catalytic amount of copper powder (0.2-0.3 microns, 4.97mmol) and dissolve in 80mL in ethanol. The reaction solution was heated under reflux for 12 hours. After the reaction was monitored by TLC, the cooled reaction solution was poured into hot water, filtered through diatomaceous earth to remove insoluble matter, and then the filtrate was acidified with diluted hydrochloric acid solution to adjust the pH to 5-6, a large amount of precipitates were separated out, and the resulting precipitated product was purified by ethanol recrystallization to obtain 1.65 g of pure gray solid product, namely intermediate 2, with a yield of 78%.
Embodiment 2
[0072] Preparation of 9(10H)-acridone (3)
[0073]
[0074] The intermediate 2-(anilino)benzoic acid (2.0 g, 9.4 mmol) prepared in Example 1 above was put into a 100 mL round bottom flask, and 10 mL of concentrated sulfuric acid was added thereto. Stir the mixture under nitrogen protection at 100°C for 6 hours. After monitoring the complete reaction by TLC, cool to room temperature, and then pour the cooled reaction solution into a large amount of ice water and stir. After the solid precipitates, it is collected by suction filtration under reduced pressure. The solid was washed several times with sodium bicarbonate solution, and dried in vacuo to obtain 1.5 g of a yellow solid product, ie Intermediate 3, with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com