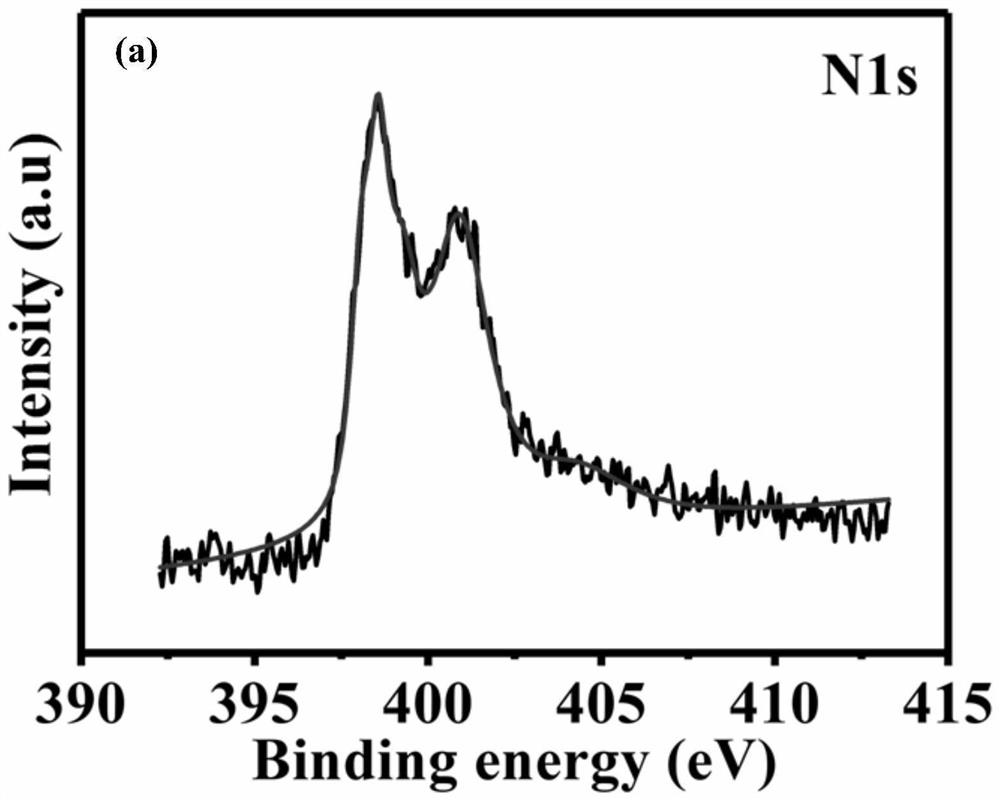
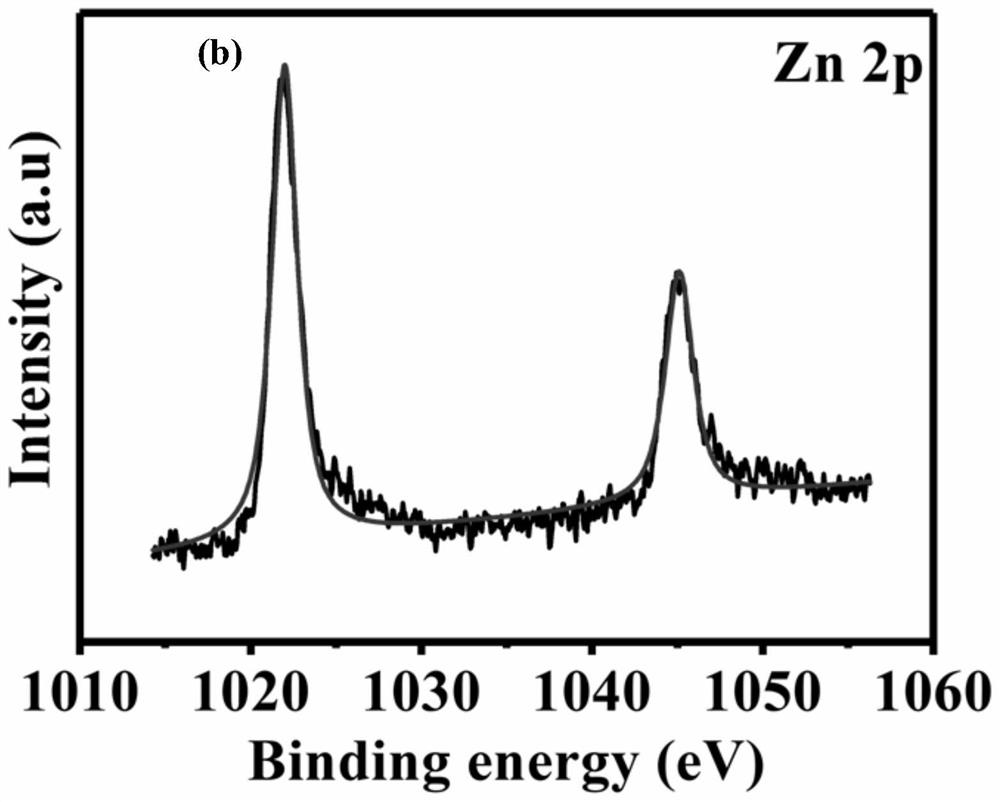
Zinc-nitrogen modified dual-carbon catalytic material, preparation method thereof and application of zinc-nitrogen modified dual-carbon catalytic material in zinc-air battery
A technology of double carbon catalytic material and porous carbon material, which can be used in fuel cell type half cells and secondary cell type half cells, carbon preparation/purification, battery electrodes, etc., and can solve the complex mixing process and complex synthesis process. , high risk factor and other problems, to achieve the effect of improving ORR capability, good conductivity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
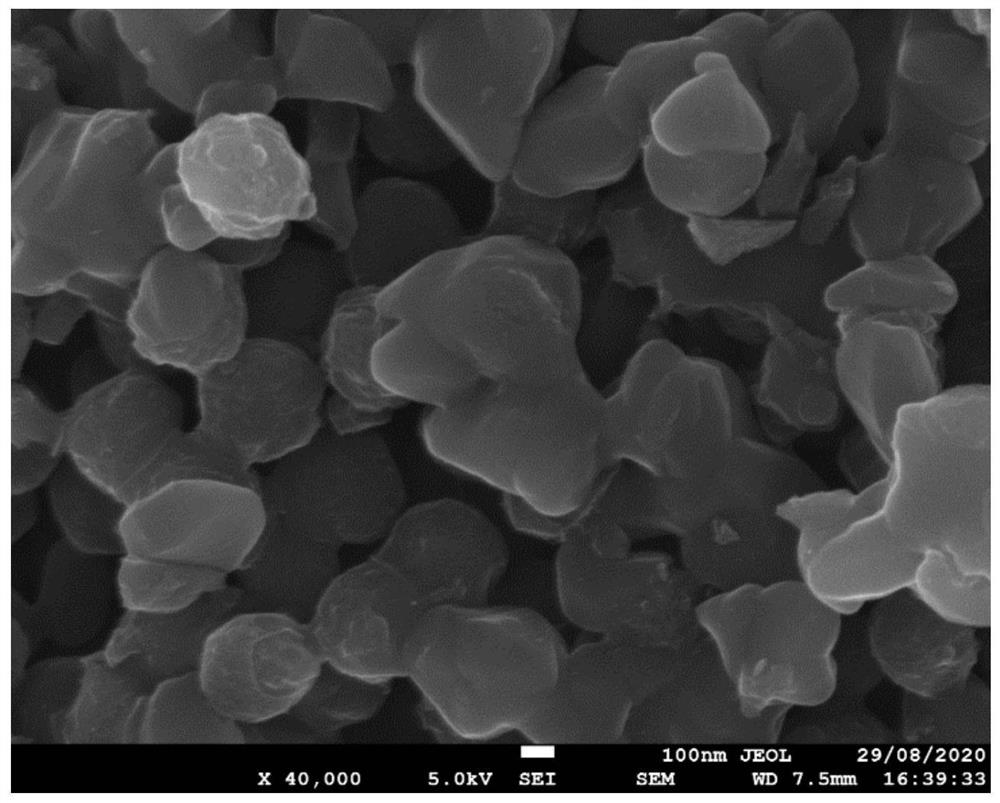
[0034] Zinc nitrate hexahydrate (1.0g) and dimethylimidazole (0.7g) were respectively dissolved in 50mL of methanol, and the dissolved dimethylimidazole was poured into zinc nitrate hexahydrate, and after stirring for 5 minutes, there was a white suspension Appeared, moved to 30 ° C environment for 12 h, centrifuged and washed to obtain ZIF-8. Put the prepared ZIF-8 in a quartz boat, place it in a tube furnace, and blow it with argon, at 5°C min -1 The heating rate was increased to 900°C, and kept at 900°C for 2h, and finally cooled to room temperature naturally to obtain a black solid powder; the powder (1g) was mixed with cyanamide (0.7g) and sucrose (0.05g), and the ball milling speed was mixed 240r min -1 , the ball milling time is 0.5h, the mixed sample is placed in a quartz boat, placed in a tube furnace, filled with argon, and heated at 5°C min -1 The heating rate was raised to 500°C for annealing, and kept at 500°C for 5 hours, then 5°C min -1 The heating rate was i...
Embodiment 2
[0037] Zinc nitrate hexahydrate (1.0g) and dimethylimidazole (0.5g) were respectively dissolved in 50mL of methanol, and the dissolved dimethylimidazole was poured into zinc nitrate hexahydrate, and after stirring for 5 minutes, there was a white suspension Appeared, moved to 40 ° C environment for 12 h, centrifuged and washed to obtain ZIF-8. Put the prepared ZIF-8 in a quartz boat, place it in a tube furnace, blow it with argon, and heat it at 3°C min -1 The heating rate was raised to 700°C, and kept at 700°C for 1h, and finally cooled to room temperature naturally to obtain a black solid powder; the powder (1g) was mixed with dibenzylcyanamide (0.5g) and fructose (0.03g), The mixing speed of the ball mill is 220 rpm -1 , the ball milling time is 1h, the mixed sample is placed in a quartz boat, placed in a tube furnace, and heated at 3°C min -1 The heating rate was raised to 400°C for annealing, and kept at 400°C for 5 hours, followed by 3°C min -1 The heating rate wa...
Embodiment 3
[0040] Zinc nitrate hexahydrate (1.0g) and dimethylimidazole (0.9g) were respectively dissolved in 50mL of methanol, and the dissolved dimethylimidazole was poured into zinc nitrate hexahydrate, and after stirring for 5 minutes, there was a white suspension appeared, moved to the environment of 20°C and stood still for 12 hours, then centrifuged and washed to obtain ZIF-8. Put the prepared ZIF-8 in a quartz boat, place it in a tube furnace, feed it with argon, and heat it at 4°C min -1 The heating rate was increased to 800°C, and kept at 800°C for 3h, and finally cooled to room temperature naturally to obtain a black solid powder; the powder (1g) was mixed with dimethylcyanamide. (1g) and glucose (0.08g), the mixing speed of the ball mill is 220r min -1 , the ball milling time is 1h, the mixed sample is placed in a quartz boat, placed in a tube furnace, and heated at 4°C min -1 The heating rate is raised to 650°C for annealing, and kept at 650°C for 3 hours, then 4°C for min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com