Construction method and application of vector vaccine for resisting infectious spleen and kidney necrosis viruses
A spleen and kidney necrosis virus, vector vaccine technology, applied in antiviral agent, virus/phage, application and other directions, can solve the problems of large amount of vaccine, poor immune effect, time-consuming and labor-intensive, etc., and achieve high biological safety and good immune effect. , easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Construction of the carrier vaccine against infectious spleen and kidney necrosis virus and the antibody produced by perch induced by injection immunization
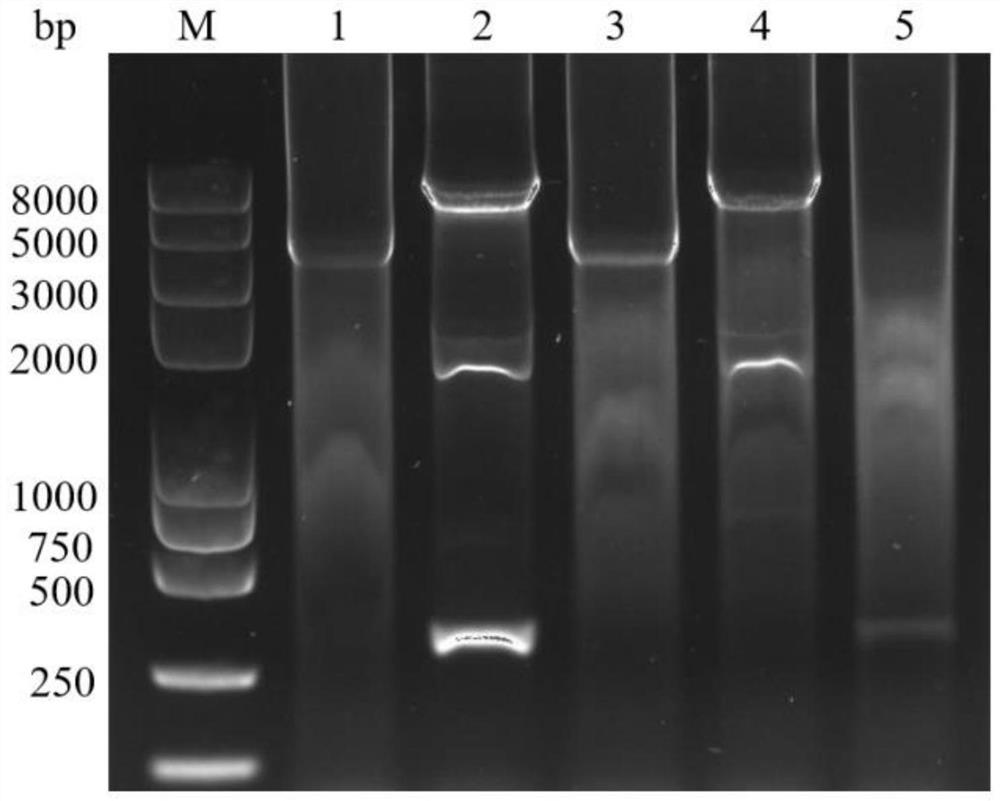
[0063] (1) Synthesize the infectious spleen and kidney necrosis virus main capsid protein gene expression cassette cmv-mcp (1997bp) controlled by the cytomegalovirus promoter as shown in SEQ ID NO: 1, clone it into the T-vector, carry out sequencing verification, and Verify that the correct plasmid is named pMD-CMV-MCP;
[0064] (2) Digest the pMD-CMV-MCP plasmid with BamHI and XbaI, recover the cmv-mcp fragment, and clone it into the same digested pFSATBac TM In Dual, the recombinant plasmid pFAST was obtained TM Dual-cmv-mcp;
[0065] (3)pFAST TMDual-cmv-mcp transformed DH10 / Bac competent cells, coated with tetracycline (10μg / ml), kanamycin (50μg / ml), gentamicin (7μg / ml), IPTG (40μg / ml) , X-gal (100μg / ml) on the LB agar culture plate; cultured at 37°C for 48 hours, picked white colonies for culture...
Embodiment 2
[0092] Example 2 The carrier vaccine BmNPV-MCP against infectious spleen and kidney necrosis virus can be transduced into the tissues of mandarin fish and express the main capsid protein of infectious spleen and kidney necrosis virus
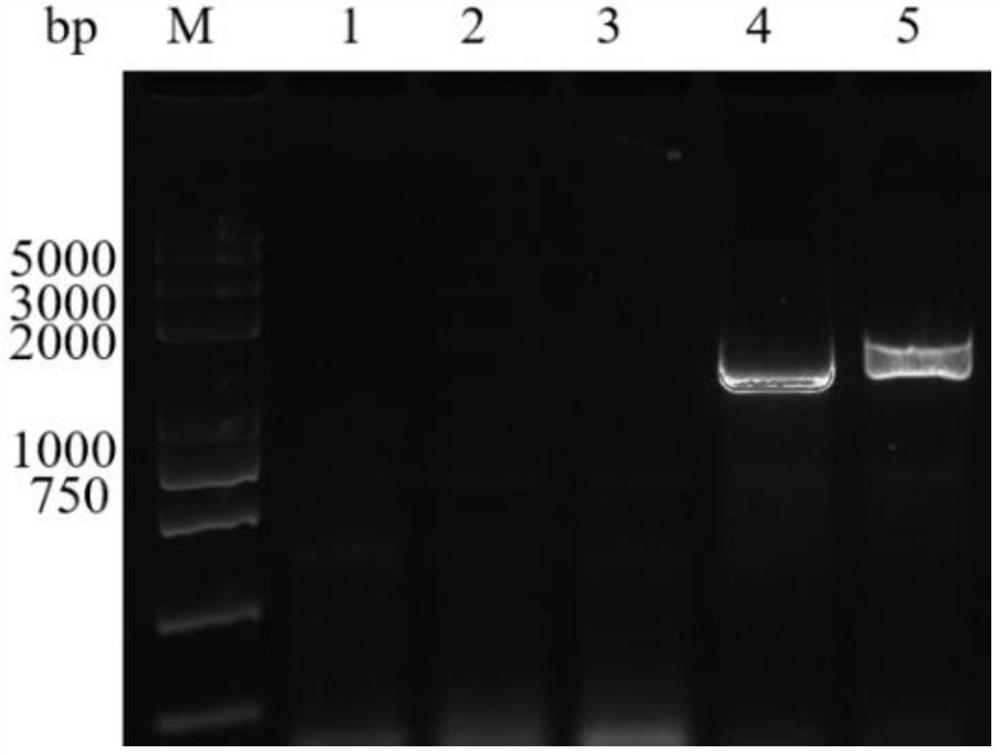
[0093] Use 8×10 9 The copied recombinant virus BmNPV-MCP was injected into mandarin fish (body length 7-13cm, weight 35-60g). One week later, a total of 0.1g of spleen and kidney tissue was taken, and the genome was extracted. Primers MCP-1 (SEQ ID NO: 4) and MCP -2 (SEQ ID NO:5) was amplified. The PCR amplification system and amplification conditions are the same as in step (4) of Example 1.
[0094] Such as Figure 6 The results shown showed that the main capsid protein gene of infectious spleen-kidney necrosis virus could be amplified by PCR from the DNA of the spleen and kidney tissues of mandarin fish injected with BmNPV-MCP, indicating that BmNPV-MCP had entered the spleen and kidney tissues of mandarin fish.
Embodiment 3
[0095] Example 3 Immunoprotective effect of immunization injection of vector vaccine against infectious spleen and kidney necrosis virus to infectious spleen and kidney necrosis virus infection
[0096] (1) Select healthy and disease-free largemouth bass from the same batch (8-12cm in length, 35-55g in weight), and raise them with oxygen at a water temperature of 19-22°C for 2 weeks.
[0097] (2) Take 4×10 7 copy / μL of BmNPV-MVP, inject 200 μL / tail from the base of the pectoral fin, and set wild BmNPV (4×10 7 copy / μL) as the negative control, and the non-immunized blank control group (200 μl of PBS per fish, 20 sea bass in total).
[0098] (3) Take 5 g of the kidney of mandarin mandarin fish infected with infectious spleen-kidney necrosis virus, add 5 mL of phosphate buffer to fully grind, centrifuge at 8000 r / min for 30 min, take the supernatant, repeat this step 4 times, and use 0.22 μm Membrane filtration, the filtrate is infectious spleen and kidney necrosis virus liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com