Salt of bile acid derivative, crystal form structure thereof, and preparation method and application of bile acid derivative salt and crystal form structure thereof
A technology of bile acid derivatives and maleate, applied in organic chemical methods, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as inconvenient development, poor solubility, and difficulty in preparation research, and achieve Good solubility and stability, improving cholestasis and reducing portal pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention also provides a method for preparing a salt of a bile acid derivative, comprising:
[0040] The compound represented by formula (I), the first solvent and the acid are mixed and reacted to obtain the salt of the bile acid derivative
[0041] The acid is an inorganic acid or an organic acid;
[0042] The inorganic acid is selected from hydrochloric acid: the organic acid is selected from methanesulfonic acid, oxalic acid, p-toluenesulfonic acid, L-tartaric acid, fumaric acid, maleic acid, preferably methanesulfonic acid, p-toluenesulfonic acid;
[0043] The first solvent is methanol, ethanol, isopropanol, isobutanol, 2-butanone, tetrahydrofuran, dichloromethane, acetonitrile, methyl tert-butyl ether, acetone, ethyl acetate, methyl formate, acetic acid One or more of isopropyl ester and n-hexane.
[0044] According to the present invention, the present invention mixes and reacts the compound represented by the formula (I), the first solvent and the a...
Embodiment 1
[0113] Example 1: Synthesis of Compound 1
[0114] The off-white solid compound 1 was prepared by the method described in Example 1 of patent CN201810930184.X.
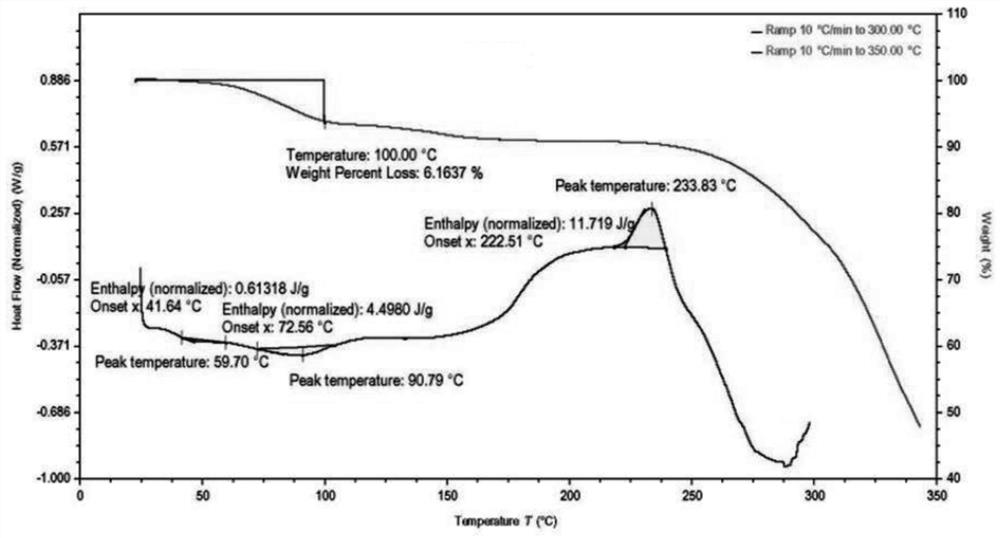
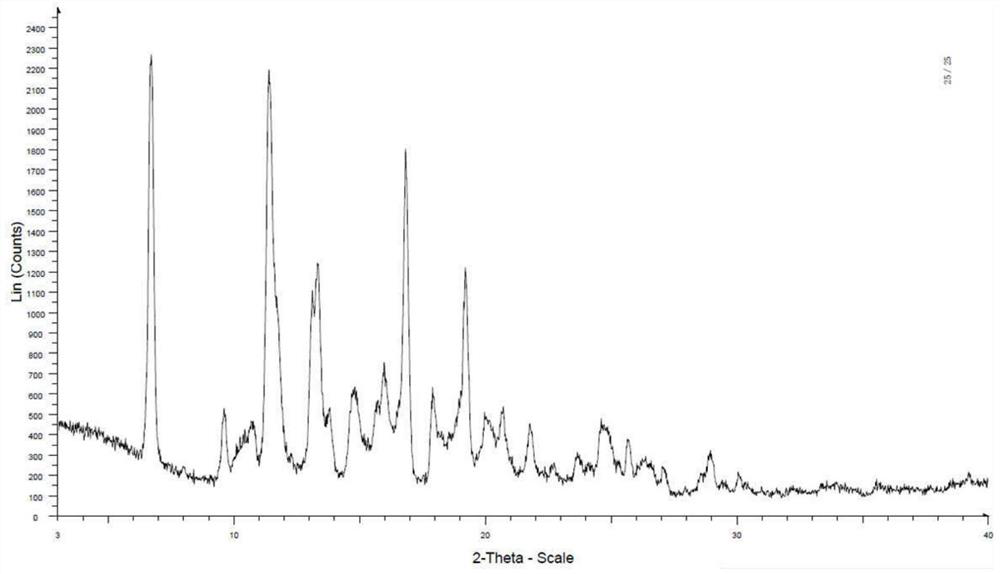
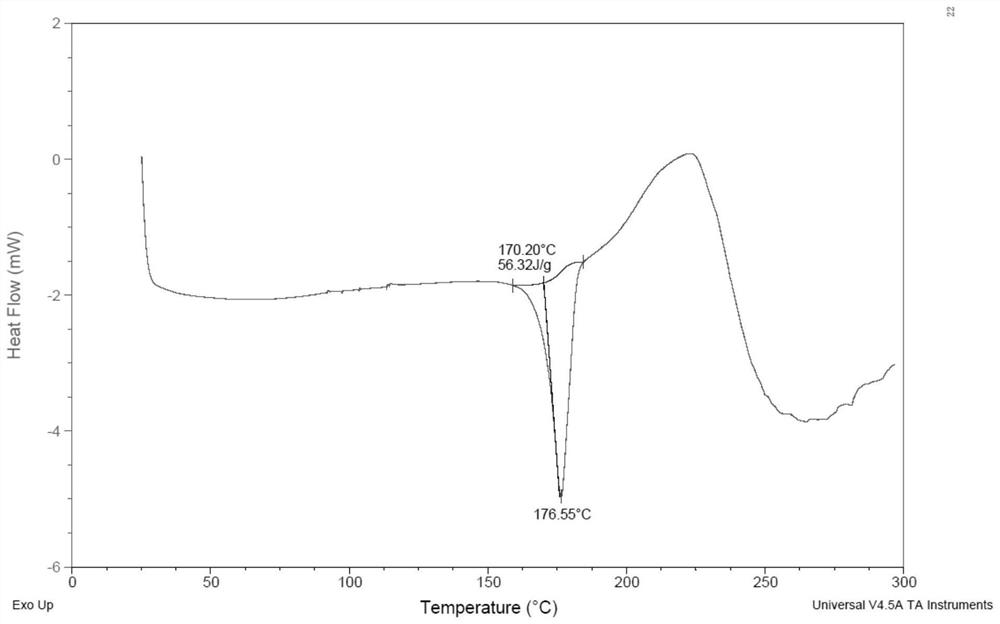
[0115] The solid was analyzed by polarized light microscope (PLM), and there was no birefringence phenomenon, and its DSC-TGA spectrum was as follows figure 1 As shown, the onset temperatures of the two endothermic peaks of DSC are located at 41.6°C and 72.6°C, respectively. TGA showed a weight loss of 6.2% before 100°C. DVS shows that under humidity conditions of 10% to 90%, moisture absorption is 1.933% to 7.0146%. Compound 1 has a low melting point and is easily hygroscopic, which is not conducive to preparation. XRPD showed that the solid was in an amorphous state, and the compound of Example 1 had a low melting point, which was unfavorable for formulation.
Embodiment 2
[0116] Example 2 Preparation of Compound 1 Hydrochloride
[0117] At room temperature, the compound of Example 1 (1.0eq.) was added to 40-60μL MeOH with stirring to dissolve it, then 1.5μL hydrochloric acid (1.0eq.) was added and stirred, no solid was precipitated, and then 100μL ACN was added, no solid was precipitated. . After the reaction flask was capped, it was slowly volatilized at room temperature to obtain a solid, and the obtained solid sample was detected. XRPD showed that the solid had no obvious diffraction peak and was in amorphous form.
[0118] UPLC-MS: (m / z): 590.3610 [M+H] + ;
[0119] 1 H-NMR (DMSO-d 6 , 400MHz, ppm): 8.98 (d, J=6.8Hz, 2H), 8.03 (d, J=6.8Hz, 2H), 6.02 (t, J=2.8Hz, 1H), 4.59 (m, 1H), 4.47 (m, 1H), 4.16 (m, 2H), 3.50 (brs, 1H), 3.14 (m, 1H), 2.42 (m, 1H), 2.16 (m, 1H), 1.91 (m, 1H), 1.81 ( m, 2H), 1.77 (m, 1H), 1.76 (m, 1H), 1.72 (m, 1H), 1.70 (m, 1H), 1.53 (m, 1H), 1.50 (m, 1H), 1.47 (m , 1H), 1.45(m, 1H), 1.42(m, 2H), 1.39(m, 1H), 1.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com