Porous condensed-ring semiconductor fluorescent polymer, fluorescent sensing film, preparation method and application thereof
A fluorescent polymer and fluorescent sensing technology, applied in the field of fluorescent sensing materials, can solve the problems that cannot further meet the requirements of fast, sensitive, and high selectivity of fluorescent sensors, and achieve enhanced monomer fluorescence intensity and high commercial application value. , the effect of suppressing the problem of fluorescence quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This embodiment provides an intermediate 2-4, the synthesis route of which is as follows:
[0050] ;
[0051] The preparation method of monomer 2-4 specifically comprises the following steps:
[0052] 1) Synthesis of compound 2-1
[0053]
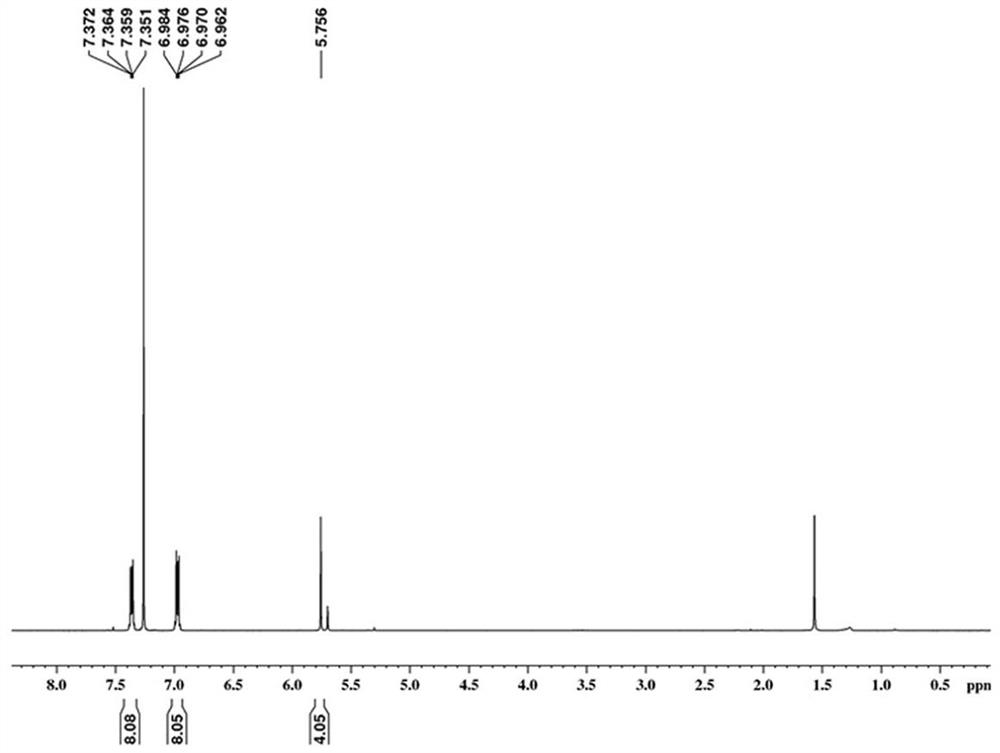
[0054] Put anthracene (3.55 g, 20.0 mmol), 1,4-benzoquinone (1.30 g, 12.0 mmol), 2,3,5,6-tetrachlorobenzoquinone (4.90 g, 20.0 mmol) into the reaction flask, add Glacial acetic acid (125 mL) was heated under reflux and stirred for 16 h. After the reaction, return to room temperature and filter under vacuum using a Buchner funnel to obtain a solid filter cake. Wash the solid with glacial acetic acid, methanol, and ether to obtain a crude product. The crude product was separated and purified by column chromatography, the eluent used was a mixed solvent of dichloromethane and ethyl acetate at a volume ratio of 50: 1, and finally yellow solid 2-1 (3.10 g, 68%) was obtained. The H NMR spectrum of compound 2-1 is as follows figure...
Embodiment 2
[0066] This example provides a porous fused-ring polymer fluorescent polymer (polymer 1), and the synthesis route of polymer 1 is as follows:
[0067] ;
[0068] The preparation method of polymer 1 specifically comprises the following steps:
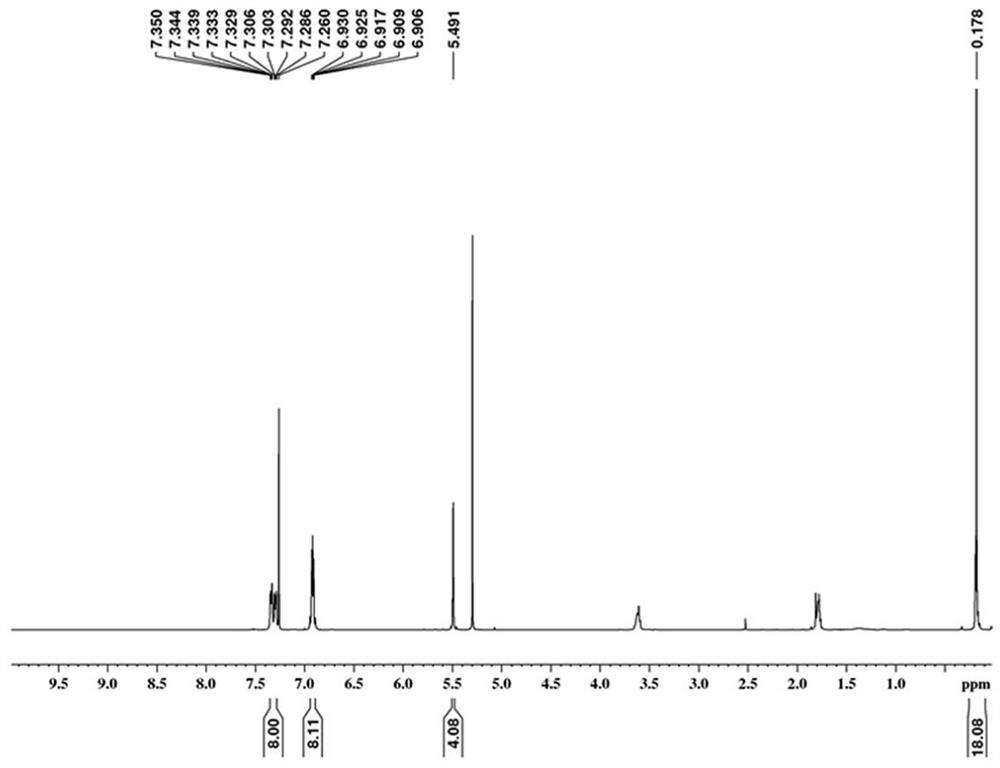
[0069] Under argon atmosphere, the compound pentadecene 2-4 (0.478 g, 1 mmol), 1,7-dibromo-3,4,9,10-perylenetetracarboxylic dianhydride (0.556 g, 1 mmol), Catalyst Pd(PPh 3 ) 4 (0.058 g, 0.05 mmol) and cuprous iodide CuI (0.010 g, 0.05 mmol) were put into the reaction flask, and the nitrogen gas was exhausted three times with a double-row tube, and ultra-dry toluene (12 mL) treated with potassium-sodium alloy was added and isopropylamine ( i -Pr 2 NH) (6 mL), heated to 130 o C stirred and reacted for two days. After the reaction was completed, the reaction mixture cooled to room temperature was poured into deionized water, extracted five times with dichloromethane, and the combined organic layers were dried with anhydrous sodium ...
Embodiment 3
[0071] This example provides a porous fused-ring polymer fluorescent polymer (polymer 2), and the synthesis route of polymer 2 is as follows:
[0072] ;
[0073] The preparation method of polymer 2 specifically comprises the following steps:
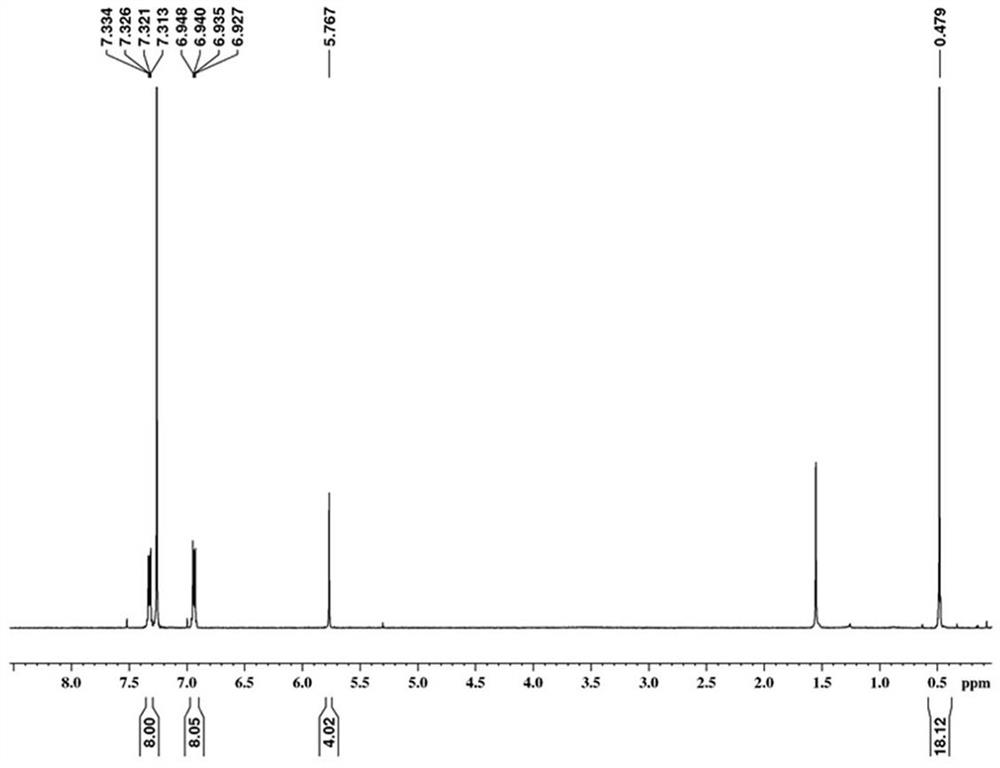
[0074] Under argon atmosphere, the compound pentadecene 2-4 (0.478 g, 1 mmol), 3,9-dibromoperylene (0.410 g, 1 mmol), catalyst Pd(PPh 3 ) 4 (0.058 g, 0.05 mmol) and cuprous iodide CuI (0.010 g, 0.05 mmol) were put into the reaction flask, and the nitrogen gas was exhausted three times with a double-row tube, and ultra-dry toluene (12 mL) treated with potassium-sodium alloy was added and isopropylamine ( i -Pr 2 NH) (6 mL), heated to 130 o C stirred and reacted for two days. After the reaction was completed, the reaction mixture cooled to room temperature was poured into deionized water, extracted five times with dichloromethane, and the combined organic layers were dried with anhydrous sodium sulfate and concentrated under vacuum ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com