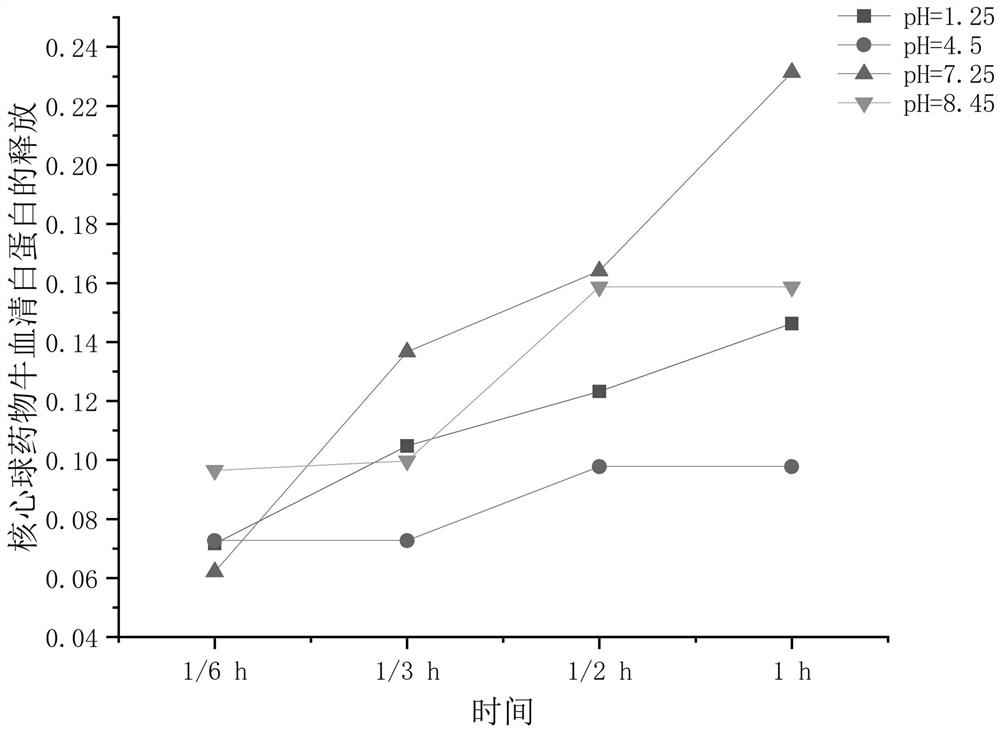
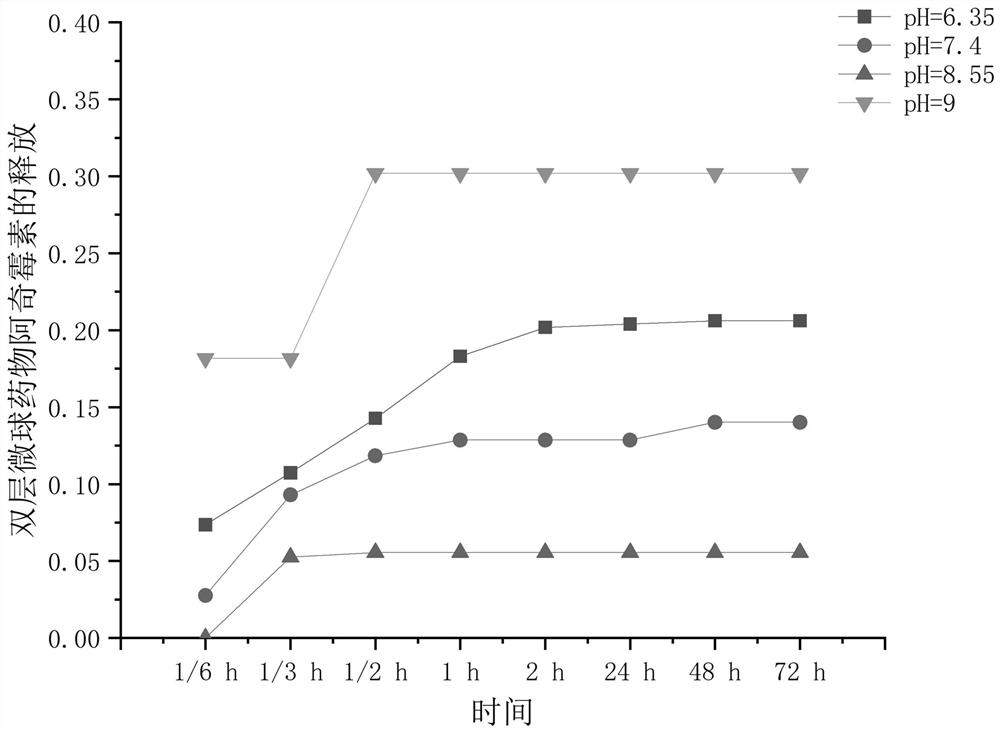
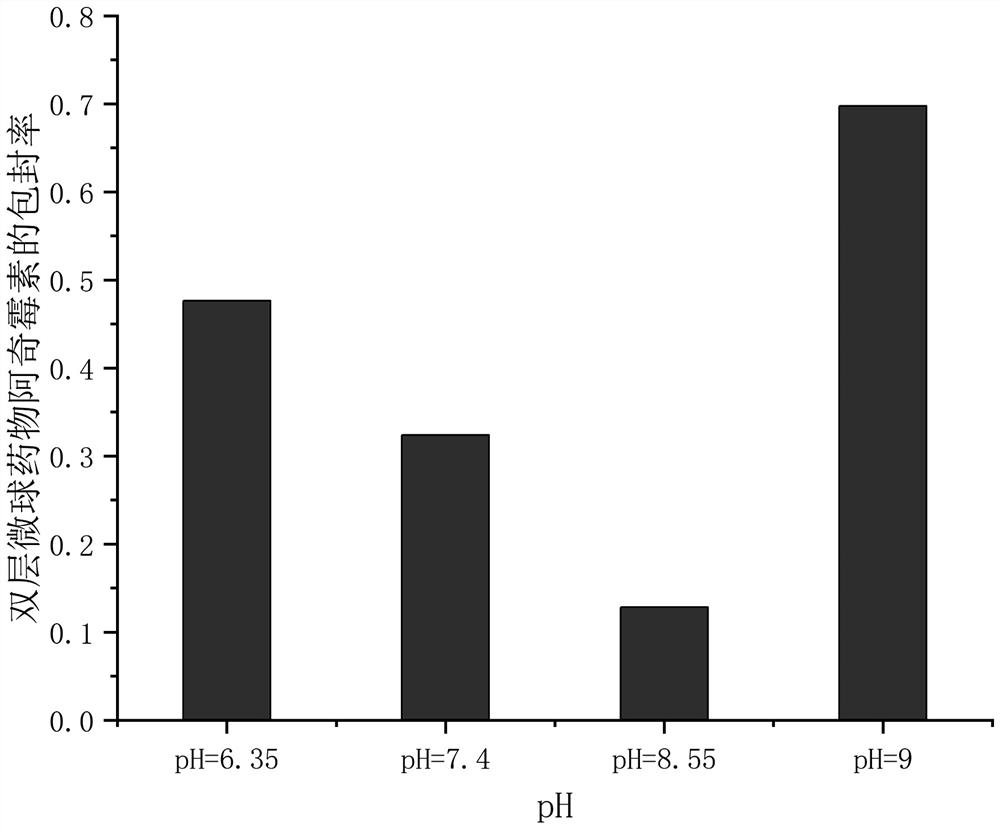
Double-sustained-release drug-loaded hydrogel dressing with semi-interpenetrating network entrapped double-layer microspheres as well as preparation method and application thereof
A semi-interpenetrating network and microsphere technology, which is applied in the field of double slow-release drug-loaded hydrogel dressings and its preparation, can solve the problems of inhomogeneous structure and inability to accurately release drugs, achieve good biocompatibility, and improve encapsulation rate, the effect of increasing the drug loading rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Orthogonal experiment is carried out the preparation of double-layer microspheres, relevant factors and corresponding levels are as follows:
[0030] Firstly, four sets of orthogonal experiments were carried out, and the morphology of the microspheres produced by the four sets of experiments was compared to determine the best formulation for the preparation of calcium alginate microspheres loaded with bovine serum albumin.
[0031] a. Measure 2mL of sodium alginate solution with a mass concentration of 4%, and load bovine serum albumin with the mass volume ratio of bovine serum albumin and sodium alginate solution at 12.5g / L for the experiment.
[0032] b. Measure 2 mL of sodium alginate solution with a mass concentration of 4%, and load bovine serum albumin with the mass volume ratio of bovine serum albumin to sodium alginate solution at 25 g / L for the experiment.
[0033] c. Measure 2mL of sodium alginate solution with a mass concentration of 2%, and loa...
Embodiment 2
[0050] Embodiment 2: Orthogonal test for the preparation of drug-loaded hydrogel, the relevant factors and corresponding levels are as follows:
[0051] Firstly, six sets of orthogonal experiments were carried out to change the content of cross-linking agent potassium persulfate and sodium thiosulfate, and the size and electron microscope morphology of the gel obtained by the six sets of experiments were compared to determine the best formula for hydrogel preparation.
[0052] a. Select 0.1 g of chitosan with a molecular weight of 500,000, 10 mL of glacial acetic acid, 0.4 mL of acrylic acid, 0.3396 mL of 2-ethyl methacrylate, and 0.095 mL of oligo(ethylene glycol) methyl ether methacrylate, 0.02 g potassium persulfate and 0.02 g sodium thiosulfate were tested;
[0053] b. Select 0.1 g of chitosan with a molecular weight of 500,000, 10 mL of glacial acetic acid, 0.4 mL of acrylic acid, 0.3396 mL of 2-ethyl methacrylate, and 0.095 mL of oligo(ethylene glycol) methyl ether metha...
Embodiment 3
[0064] Embodiment 3: the preparation of the hydrogel loaded with double-layer microspheres, the specific experimental process is as follows:
[0065] Put 0.1 g chitosan (molecular weight: 500,000, degree of deacetylation: 95%) in a beaker, take 10 mL of 1% acetic acid aqueous solution, stir until completely dissolved; weigh 0.4 mL of acrylic acid, 0.3396 mL of methacrylic acid 2-Ethyl ester and 0.095mL oligo(ethylene glycol) methyl ether methacrylate monomer were added to the chitosan solution, and stirred thoroughly for 30 min to make it evenly mixed.
[0066] The mixture of 0.03 g potassium persulfate and 0.03 g sodium thiosulfate was dissolved in 0.2 mL of aqueous solution, and the mixed solution was added into the mixed solution of chitosan and monomer, stirred for 20 min to fully dissolve, and 0.2 ~0.4 mL gentamicin sulfate and add 0.02~0.05 g double-layer microspheres.
[0067] The mixed solution was poured into the mold, nitrogen gas was continuously passed for 15 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com