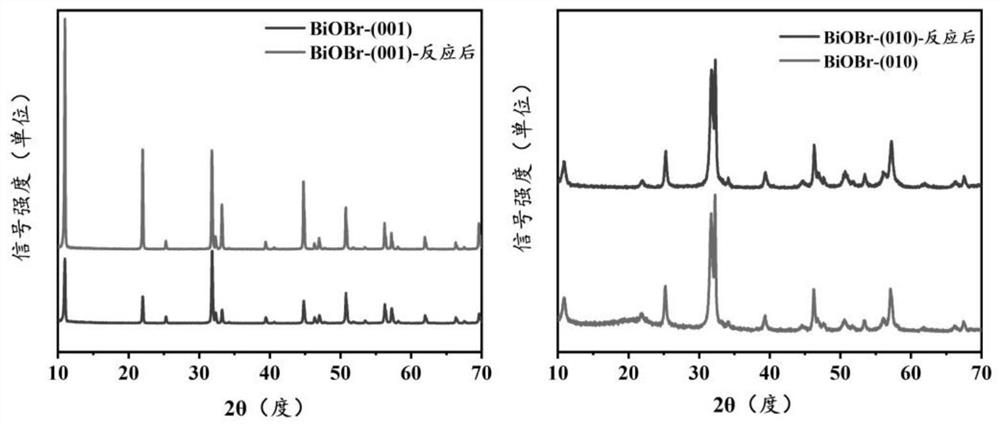
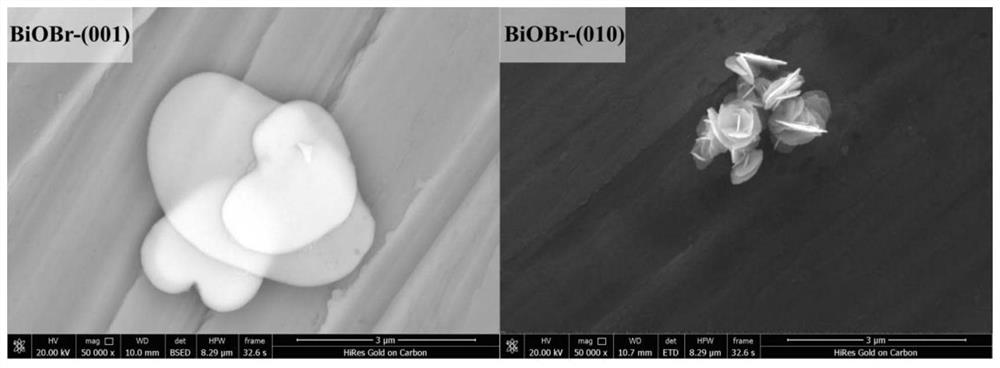
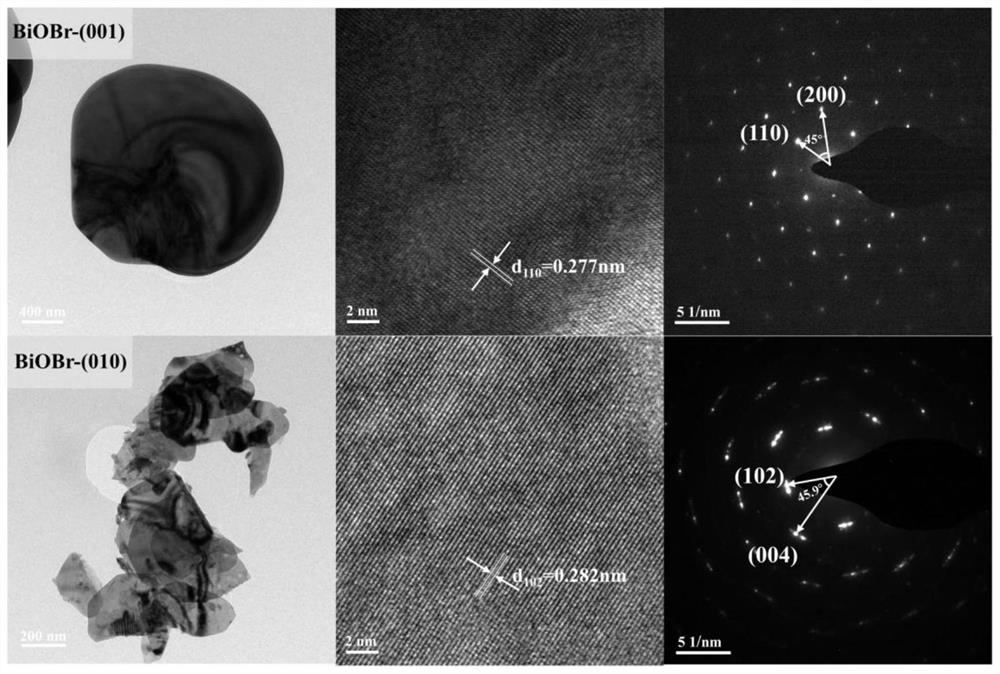
Application of crystal face controllable bismuth oxybromide catalyst in selective oxidative degradation of pollutants
A bismuth oxybromide and catalyst technology are applied in the application field of crystal face-controllable bismuth oxybromide catalyst in the selective oxidation and degradation of pollutants, and can solve the problem of pH applicable range, unknown resistance to environmental interference, difficult advanced oxidation water treatment Process, poor catalytic activity and other problems, to achieve the effect of huge application potential, low leakage rate, strong anti-interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The invention provides a method for preparing a bismuth oxybromide catalyst with controllable crystal planes, comprising: S1) mixing a bismuth source, a potassium source and nitric acid in water, adjusting the pH value of the mixed solution to weak acidity, and obtaining a precursor solution; S2) heating the precursor solution to carry out a hydrothermal reaction to obtain a bismuth oxybromide catalyst with controllable crystal facets.
[0040] Among them, the present invention has no special limitation on the sources of all raw materials, which can be commercially available.
[0041] Mix the bismuth source, the potassium source and nitric acid in water; the bismuth source is preferably an inorganic bismuth salt, more preferably bismuth nitrate; the potassium source is preferably an inorganic potassium salt, more preferably potassium halide, and more preferably potassium bromide The molar ratio of the bismuth source to the potassium source is preferably 1: (1-1.5), more...
Embodiment 1
[0052] Add 1.94 g of bismuth nitrate pentahydrate into the beaker, add 10 mL of 1 mol / L nitric acid solution, and stir to dissolve.
[0053] Take another clean beaker, add 0.595g potassium bromide, add 50mL deionized water, stir to dissolve.
[0054] Under continuous magnetic stirring, the bismuth nitrate solution was slowly added dropwise to the potassium bromide solution. After mixing and stirring, the pH value of the mixed solution was adjusted to 6 with 2 mol / L sodium hydroxide solution to obtain a milky white precursor solution.
[0055] The obtained milky white precursor solution was transferred to a polytetrafluoroethylene reactor liner with a capacity of 100 ml. After putting on a stainless steel kettle cover, put it in an oven and gradually raise the temperature to 160 degrees Celsius, carry out a hydrothermal reaction for 12 hours, and then cool it down naturally. Dry in a blast drying oven at 60 degrees Celsius to obtain BiOBr material powder, which is denoted as B...
Embodiment 2
[0069] Embodiment 2: BiOBr-PMS system is used for actual water sample and multiple pollutant treatment
[0070] Add the BiOBr-{010} material powder obtained in Example 1 into a 10mg / L bisphenol solution, and then add 5mM / L sodium carbonate, sodium bicarbonate, sodium chloride, sodium sulfate and potassium nitrate to test The effect of common anions in the system, Figure 7 is the degradation rate curve of bisphenol A in the BiOBr-{010} / PMS system in the presence of common anions in the aqueous environment. Depend on Figure 7 It can be seen that within 20 minutes, different anions common in the water environment have little effect on the degradation of bisphenol A in the BiOBr-PMS system, among which carbonate and bicarbonate can significantly improve the removal efficiency of bisphenol A, which may be Associated with the hydrolysis of carbonate and bicarbonate resulting in a weakly alkaline environment. Chloride ions, nitrate ions and sulfate ions had little effect on the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Kinetic constant | aaaaa | aaaaa |
| Kinetic constant | aaaaa | aaaaa |
| Kinetic constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com