Application and preparation method of bimetallic catalyst for synthesizing dimethyl 1,2-cyclohexanedicarboxylate from dimethyl phthalate
A technology of dimethyl cyclohexanedicarboxylate and dimethyl phthalate, which is applied in the field of catalyst preparation, can solve the problems of decreased catalytic activity of the catalyst, uniform dispersion of active components, and inability to give full play to the mutual coordination effect, and the like, Achieving the effect of small mass transfer limit, simple and easy method, enhanced electron transport characteristics and chemical reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
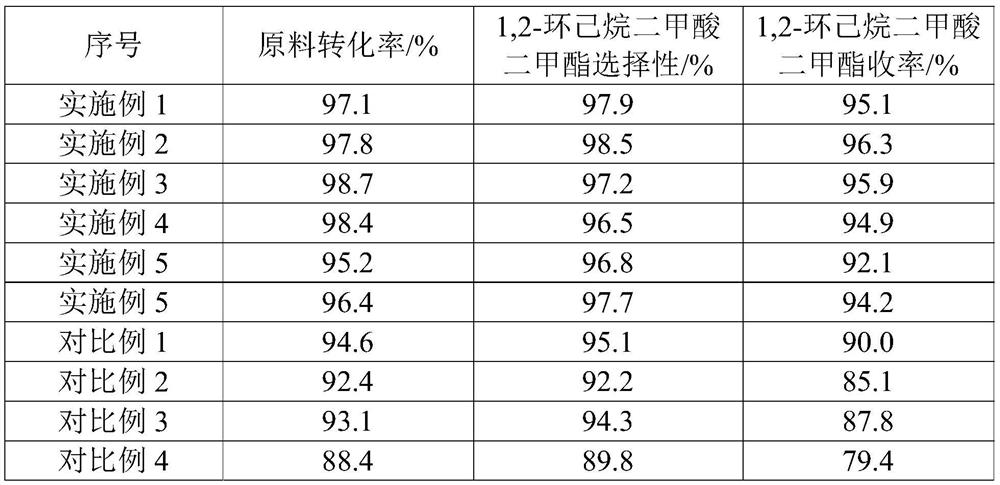
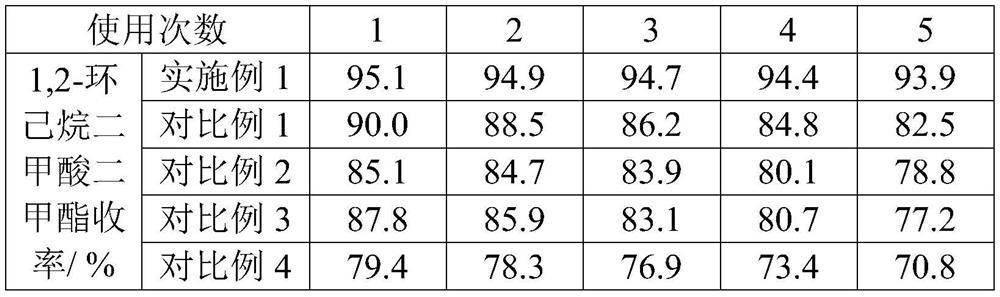
Examples
Embodiment 1
[0023] (1) Preparation of nitrogen-doped carbon nanotube support
[0024] Grind and grind 4g of melamine salt into a porcelain boat, wrap 2g of Fe / SBA-15 molecular sieve catalyst on the melamine, pass in argon, the flow rate of argon is 80ml / min, heat to 650°C and keep it for 1 hour, A black powder was obtained. Then the black powder was soaked with 2mol / L hydrofluoric acid for 4 hours to remove the catalyst, and finally washed with deionized water until neutral, and dried at 70°C to obtain nitrogen-doped carbon nanotubes (N-CNTs).
[0025] (2) Preparation of bimetallic catalysts by galvanic displacement method
[0026] The nitrogen-doped carbon nanotubes prepared in step (1) were dispersed in the nickel chloride solution, and the nickel chloride consumption was calculated as 10% of the mass of the carrier based on nickel, stirred at room temperature for 3 hours, and then added dropwise with KBH under stirring 4 Aqueous solution (the molar ratio of KBH4 to nickel is 4:1), fi...
Embodiment 2
[0030] In embodiment 1, palladium chloride is changed to use ruthenium chloride.
[0031] Nitrogen-doped carbon nanotubes are dispersed in nickel chloride solution, the amount of nickel chloride is calculated as nickel accounts for 10% of the carrier mass, stirred at room temperature for 3 hours, and then KBH is added dropwise under stirring 4 Aqueous solution (KBH 4 The molar ratio to nickel is 4:1), filtered and washed until neutral, then dispersed in deionized water, heated to 100°C, and then added dropwise with ruthenium chloride aqueous solution (the amount of ruthenium chloride is based on the molar ratio of ruthenium to nickel being 2 : 1 calculation), and then stirred for 4 hours to ensure that all ruthenium ions were replaced. After the galvanic couple was replaced, after the slurry was cooled to normal temperature, 4 ml of hydrochloric acid (37% concentration) was added dropwise. Finally, the black solid was filtered out and washed with deionized water until neutra...
Embodiment 3
[0034]In Example 1, the amount of nickel chloride is calculated as nickel accounts for 13% of the mass of the carrier.
[0035] Other operations are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com