Method for co-processing arsenic sulfide slag and arsenic-containing smoke in copper smelting
A co-processing and arsenic sulfide technology, applied in the field of metallurgy, can solve the problems of poor stability of arsenic-containing solid waste, large consumption of iron powder, long process flow, etc., achieve efficient removal and stable solidification, less loss of valuable metals, The effect of high arsenic removal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
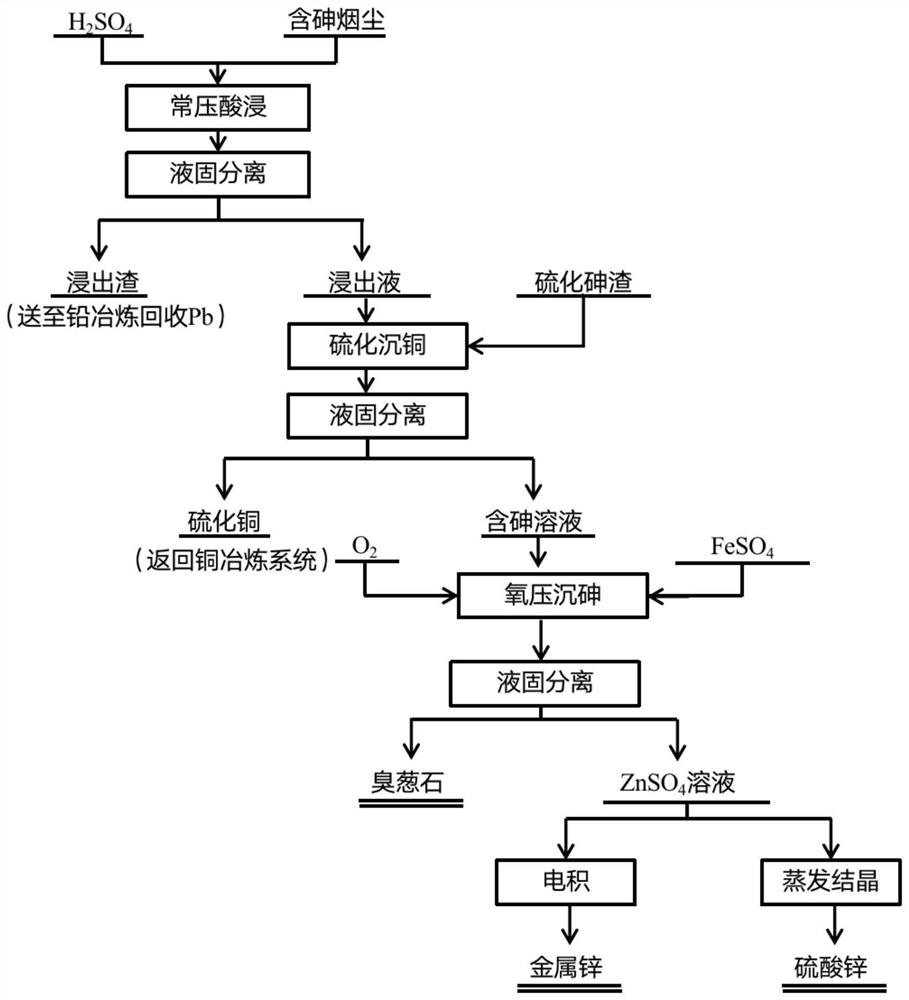
[0051] Taking a domestic arsenic-containing smoke as an example, it includes the following main components in terms of mass percentage: Pb 8.92%, As 15.6%, Fe 1.59%, Cu 2.58%, Zn 29.89%.
[0052] The arsenic sulfide slag includes the following main components in terms of mass percentage: arsenic: 25.8%, copper: 0.21%, selenium: 0.11%, sulfur: 34.7%.
[0053] Weigh 20g of arsenic-containing dust into a three-neck flask, add 140ml of H 2 SO 4 , stirring speed 300r / min, H 2 SO 4 The leaching experiment was carried out under the conditions of concentration 1mol / L, leaching temperature 50℃, and leaching time 3h. After leaching, remove the slurry, wash with water, filter and separate to obtain leaching slag and acid leaching solution, the leaching rate of arsenic is 98.72%, the leaching rate of copper is 99.40%, the leaching rate of zinc is 95.73%, and the concentration of each element in the leaching solution is Zn 42.39g / L, Cu 3.80g / L, As 23.69g / L, Fe 0.26g / L, Pb 34.71ppm, and...
Embodiment 2
[0057] Taking the domestic arsenic-containing dust as an example, it includes the following main components in terms of mass percentage: Pb 7.35%, As12.56%, Fe 3.2%, Cu 3.52%, Zn 22.10%.
[0058] The arsenic sulfide slag includes the following main components in terms of mass percentage: arsenic: 32.4%, copper: 1.13%, selenium: 0.19%, sulfur: 48.5%.
[0059] Weigh 20g of arsenic-containing fumes into a three-necked flask, add 60ml of H 2 SO 4 , stirring speed 500r / min, H 2 SO 4 The leaching experiment was carried out under the conditions of concentration 0.3mol / L, leaching temperature 85℃, and leaching time 2h. After leaching, remove the slurry, wash with water, filter and separate to obtain leaching slag and acid leaching solution, the leaching rate of arsenic is 95.03%, the leaching rate of copper is 90.35%, the leaching rate of zinc is 92.11%, and the concentration of each element in the leaching solution is Zn 74.02g / L, Cu 11.56g / L, As 47.73g / L, Fe 0.44g / L, Pb 32.13ppm...
Embodiment 3
[0062] Taking domestic arsenic-containing dust as an example, it includes the following main components in terms of mass percentage: Pb 6.65%, As 8.78%, Fe 5.64%, Cu 10.21%, Zn 5.65%.
[0063] The arsenic sulfide slag includes the following main components in terms of mass percentage: arsenic: 15.62%, copper: 2.69%, selenium: 1.30%, sulfur: 23.56%.
[0064] Weigh 20g of arsenic-containing dust into a three-neck flask, add 200ml of H 2 SO 4 , stirring speed 700r / min, H 2 SO 4 The leaching experiment was carried out under the conditions of concentration 0.5mol / L, leaching temperature 50℃, and leaching time 2h. After leaching, remove the slurry, wash with water, filter and separate to obtain leaching slag and acid leaching solution, the leaching rate of arsenic is 96.49%, the leaching rate of copper is 96.54%, the leaching rate of zinc is 97.21%, and the concentration of each element in the leaching solution is Zn 5.78g / L, Cu10 .38g / L, As 8.92g / L, Fe 1.34g / L, Pb 21.65ppm, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com