Antibacterial composite coating as well as preparation method and product thereof
A composite coating, antibacterial coating technology, applied in coatings, pharmaceutical formulations, catheters, etc., to achieve good lubricity, reduce the development of bacterial resistance, and good firmness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0131] These groups or molecules with antibacterial function mainly undergo esterification reaction, transesterification reaction, amidation reaction, click reaction, protonation reaction, ionization reaction, ring opening reaction, addition reaction, elimination reaction, aldol reaction, initiation Polymerization etc. are introduced. For example, the substitution of adamantanoyl chloride and PHMG (polyhexamethyleneguanidine hydrochloride) introduces guanidinium salt groups; the quaternization reaction of 6-bromoβ-cyclodextrin and aminoethanol introduces quaternary ammonium groups; bovine serum albumin and allyltrimethylammonium bromide click reaction to introduce serum proteins, etc. In some embodiments of the present invention, the functionalized guest molecule and / or host molecule is a functionalized host molecule, more preferably a guanidinium salt modified cyclodextrin and / or a quaternary ammonium salt modified cyclodextrin, Cyclodextrins usually contain 6 to 12 D-glucop...
preparation example 1
[0140] Preparation example 1: Preparation of methacrylate hydroxyethyl acrylate
[0141] In a 250mL three-necked flask equipped with mechanical stirring, add 26.0g of hydroxyethyl methacrylate (0.2mol), 100mL of dry dichloromethane and 25.3g of triethylamine (0.25mol), and slowly drop it with a constant pressure dropping funnel Add 21.7g of acryloyl chloride (0.24mol), react at 0°C for 0.5 hours, raise the temperature to 28°C for 10 hours, filter the crude product, separate and purify the crude product by column chromatography, and obtain 30.2g of yellow flaky crystals after drying. Ethyl acrylate is shown in structural formula 1, and the yield is 82%. 1 H NMR (CDCl 3 )δ:6.44(m,1H,CH 2 =CH),6.10(m,2H,CH 2 =CH, CH=CH), 5.84 (m, 1H, CH 2 =CH), 5.58(m, 1H, CH=CH), 4.38(m, 4H, COOCH 2 CH 2 OCO), 1.94(s, 3H, CH 3 ).
[0142]
preparation example 2
[0143] Preparation example 2: Preparation of benzophenone-2-aminopropyl ethyl methacrylate (IIa)
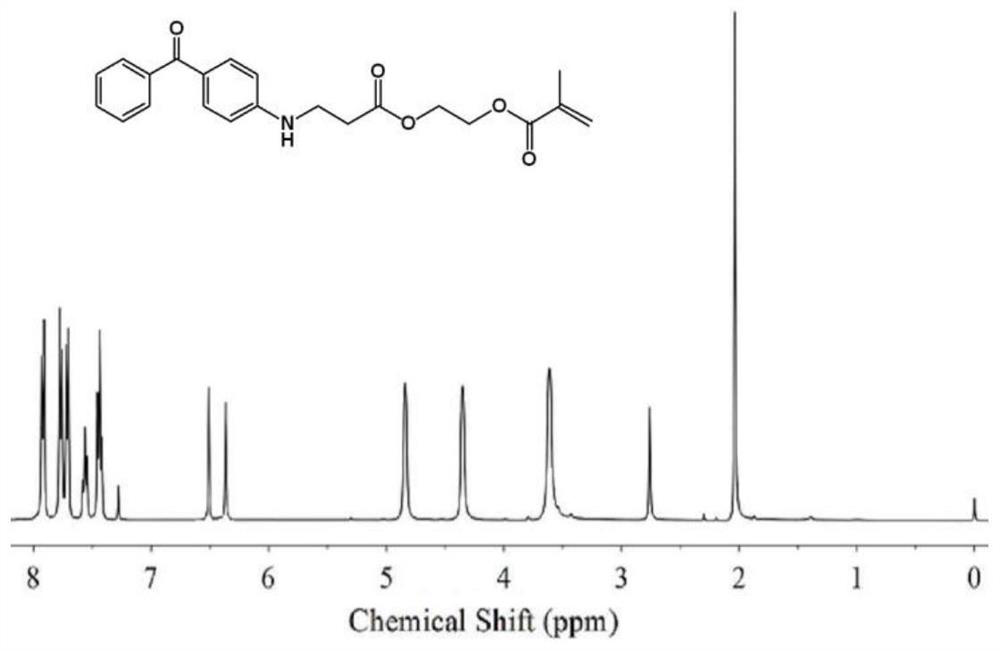
[0144] Add 18.4g (0.1mol) of methacrylate hydroxyethyl acrylate prepared in Preparation Example 1 into a 250mL three-necked flask, add 100mL of ethanol and stir, and after it is completely dissolved, add 19.7g (0.1mol) of 4-aminodimethacrylate Benzophenone was heated to 60°C for 14 hours. The reaction solution was spin-dried, and recrystallized from ethanol and ethyl acetate to obtain 34 g of a light yellow solid with a yield of 90%. 1 HNMR (CDCl 3 )δ: 7.32-7.87 (m, 9H, benzene ring), 6.42-6.48 (m, 2H, CH 2 =CH), 4.78(s,2H,-CH 2 -CH 2 -O),4.32(s,2H,-CH 2 -CH 2 -O), 3.62(m, 2H, NH-CH 2 -CH 2 -), 2.58 (m, 2H, NH-CH 2 -CH 2 -), 2.01(s, 3H, CH 3 ), see the specific map figure 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com