Method for preparing polysubstituted benzofuran-4-formic acid compound through ruthenium catalysis
A benzofuran, multi-substitution technology, applied in the field of ruthenium-catalyzed preparation of multi-substituted benzofuran-4-carboxylic acid compounds, can solve the problems of low universality, inapplicability of carboxybenzofuran, and no synthetic technology reported , to achieve the effect of convenient reaction operation, good application prospect and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
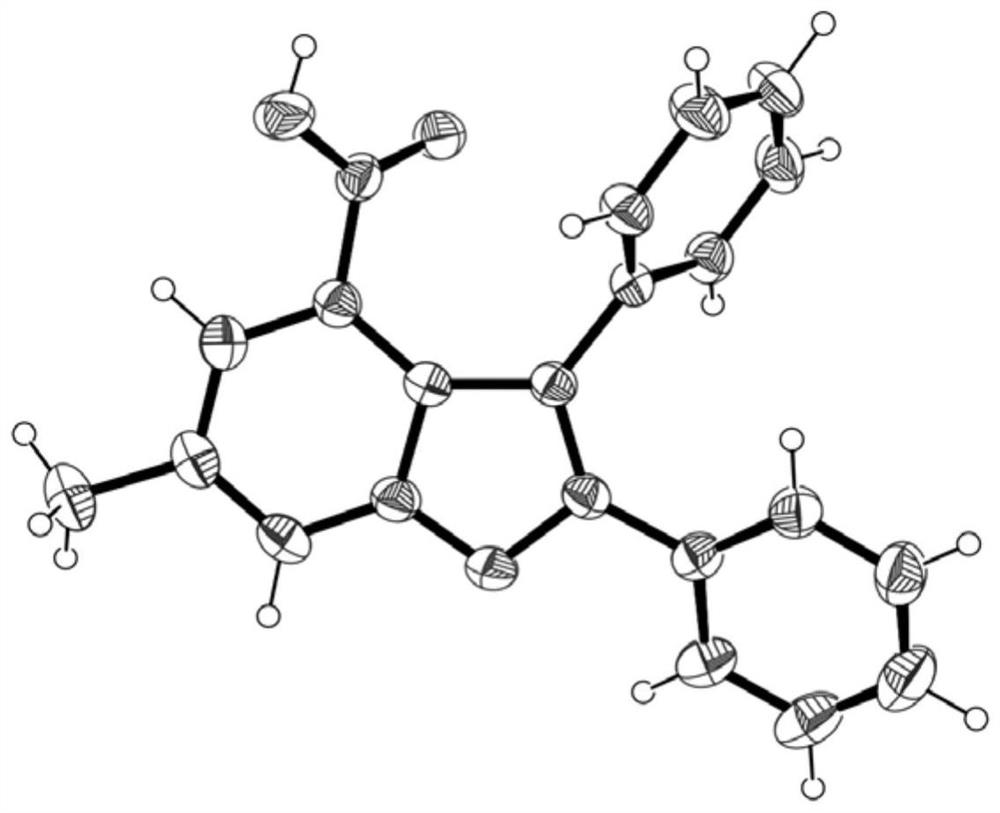
[0042] In a 10mL reaction tube with a branch, add a magnetic stirrer, p-cymene dichloride ruthenium dimer (4.9mg, 0.008mmol), 3-hydroxy-5-methylbenzoic acid (73.2mg, 0.48mmol ), toluene (71.3mg, 0.4mmol), magnesium acetate tetrahydrate (21.4mg, 0.10mmol) and 0.5mL GVL, cover the upper opening of the reaction tube and leave its branch open to the air, then stir at 100 ° C React for 20 hours. After the reaction was completed, ethyl acetate was added, filtered through a thin layer of diatomaceous earth, saturated sodium chloride solution was added, ethyl acetate extracted and separated, and the organic phase was dried with anhydrous sodium sulfate and concentrated. Using petroleum ether and ethyl acetate as the eluent, use silica gel column chromatography to separate, rotary evaporate and suck dry, and obtain a white solid (95.7mg, yield 73%) which is the product 6-methyl-2,3-di Phenylbenzofuran-4-carboxylic acid.
[0043] The structure of the obtained product is determined by ...
Embodiment 2
[0045] In a 10mL reaction tube with a branch, add a magnetic stirrer, p-cymene dichloride ruthenium dimer (4.9mg, 0.008mmol), 3-hydroxy-5-methylbenzoic acid (73.2mg, 0.48mmol ), toluene (71.3mg, 0.4mmol), magnesium acetate tetrahydrate (21.4mg, 0.10mmol) and 0.5mL GVL, cover the upper opening of the reaction tube and leave its branch to connect to the oxygen balloon, and then at 100 ° C The reaction was stirred for 20 hours. After the reaction was completed, ethyl acetate was added, filtered through a thin layer of diatomaceous earth, saturated sodium chloride solution was added, ethyl acetate extracted and separated, and the organic phase was dried with anhydrous sodium sulfate and concentrated. Using petroleum ether and ethyl acetate as the eluent, use silica gel column chromatography to separate, rotary evaporate and suck dry, and obtain a white solid (93.5 mg, yield 71%) which is the product 6-methyl-2,3-di Phenylbenzofuran-4-carboxylic acid.
Embodiment 3
[0047] In a 10mL reaction tube with a branch, add a magnetic stirrer, hydrated ruthenium trichloride (0.016mmol, 3.3mg), 3-hydroxy-5-methylbenzoic acid (73.2mg, 0.48mmol), toluene ( 71.3mg, 0.4mmol), anhydrous magnesium acetate (21.4mg, 0.10mmol) and 0.5mL GVL, cover the upper opening of the reaction tube and leave the branch open to the air, then stir and react at 100°C for 24 hours. After the reaction was completed, ethyl acetate was added, filtered through a thin layer of diatomaceous earth, saturated sodium chloride solution was added, ethyl acetate extracted and separated, and the organic phase was dried with anhydrous sodium sulfate and concentrated. Using petroleum ether and ethyl acetate as the eluent, use silica gel column chromatography to separate, rotary evaporate and suck dry, and obtain a white solid (76.0 mg, yield 58%) which is the product 6-methyl-2,3-di Phenylbenzofuran-4-carboxylic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com