Conjugated polymer based on thiophene and benzothiadiazole and preparation method of conjugated polymer
A technology of benzothiadiazole and conjugated polymer, which is applied in the field of donor polymer and its preparation, can solve the problems of harm to human body and environment, expensive synthesis cost, toxic by-products, etc., and achieve good chemical stability and Thermal stability, low synthesis cost, simple and mature synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, preparation based on the conjugated polymer of thiophene and benzothiadiazole
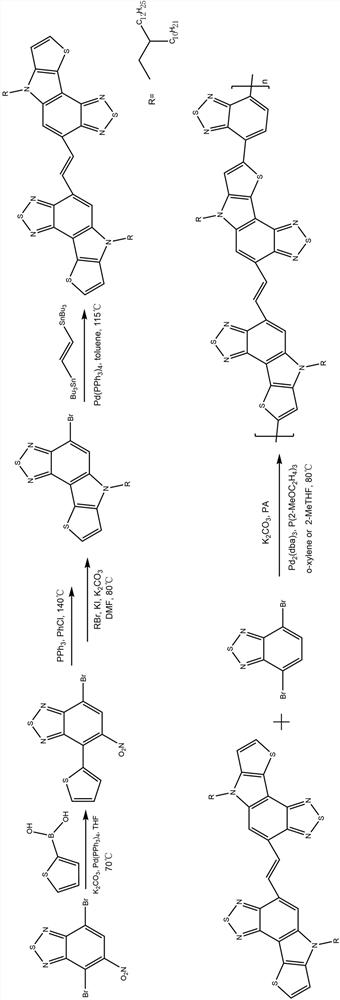
[0040] This example provides a conjugated polymer based on thiophene and benzothiadiazole, and its synthetic route can be found in figure 1 .
[0041] 1.1. Preparation of monomer M1
[0042] The preparation of monomer M1 specifically comprises the following steps:
[0043] (a) Synthesis of intermediate compound A
[0044] The structural formula of intermediate compound A is
[0045]
[0046] Under argon atmosphere, 4,7-dibromo-5-nitrobenzo[c][1,2,5]thiadiazole (5mmol), 2-thiopheneboronic acid (5.5mmol) and tetrahydrofuran (15mL) Add it into a pressure-resistant tube, and after bubbling under the liquid for 20 minutes, add 2M potassium carbonate aqueous solution (10 mL) and tetrakis(triphenylphosphine)palladium (0.025 mmol), remove oxygen from the liquid, and seal it. After reacting at 70°C for 24 hours, extract with water and dichloromethane, dry over anhydrous magnesiu...
Embodiment 2
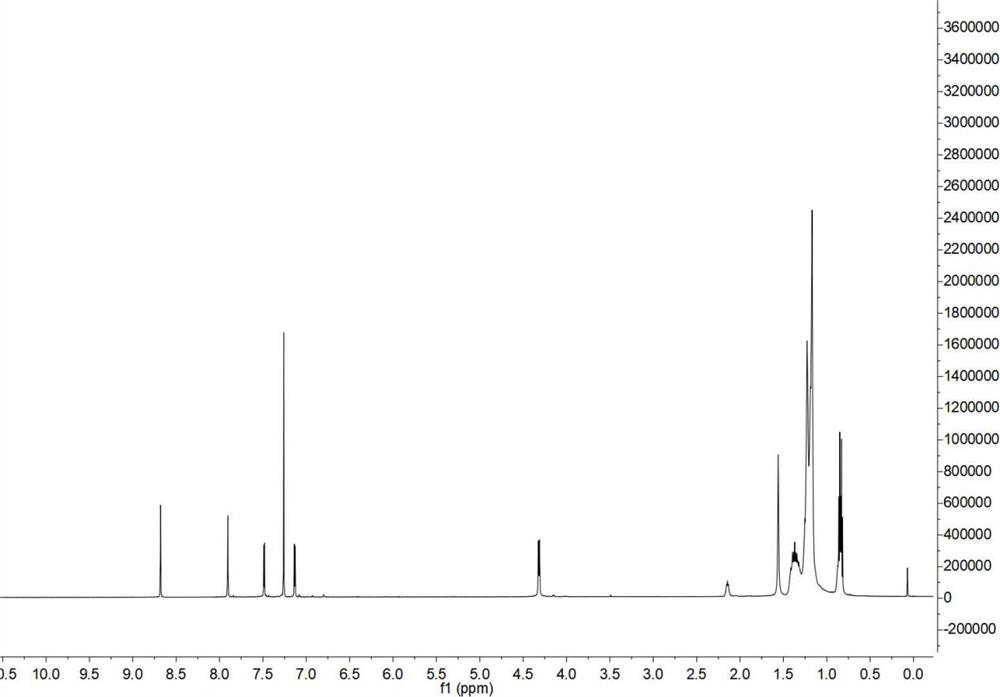
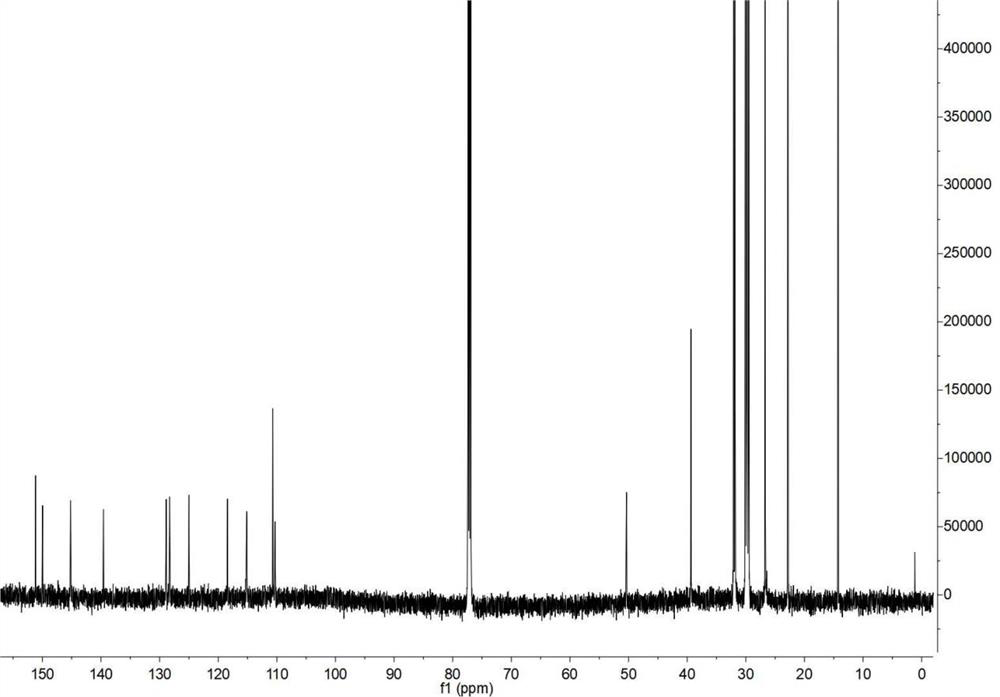
[0061] Embodiment 2, the gel permeation chromatography of polymer P, ultraviolet absorption spectrum, electrochemical properties and thermal stability 2.1, the gel permeation chromatography of polymer P
[0062] Figure 5 The number average molecular weight measured by gel permeation chromatography (GPC) of polymer P is 6179, the weight average molecular weight is 51856, and the distribution coefficient PDI is 5.01.
[0063] 2.2. UV absorption spectrum of polymer P
[0064] Image 6 The ultraviolet absorption spectrum of polymer P in chlorobenzene solution and film is given. It can be seen that the polymer has two absorption peaks. The maximum absorption peak position of its film is around 648nm, and the maximum absorption peak position of the solution is at 662nm It has a wide absorption range of ultraviolet and visible light, indicating that polymer P has a certain application potential in organic field effect transistors. The onset absorption wavelength of the polymer fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com