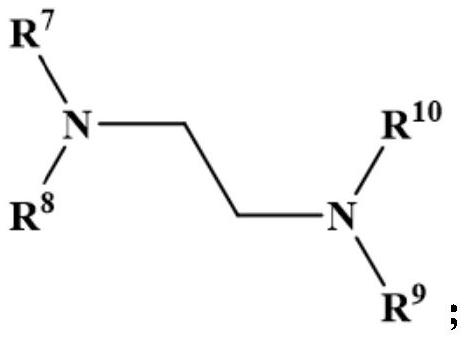
Aromatization method of nitrogen-containing heterocyclic compound
A nitrogen heterocyclic compound, aromatization technology, applied in the field of aromatization of nitrogen-containing heterocyclic compounds, can solve the problems of large amount of catalyst, long reaction time, easy over-oxidation, etc., achieve high conversion rate and selectivity, post-treatment The process is simple and the effect of improving biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
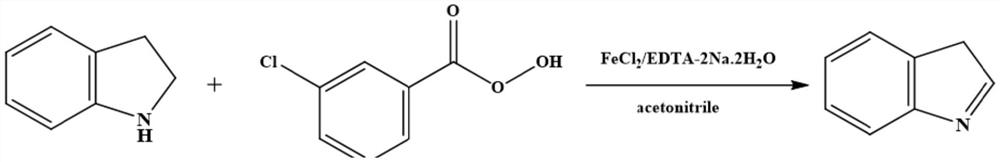
[0037] Synthesis of 3-indoline
[0038] Add 80g of acetonitrile, catalyst (2.13mg, 0.0168mmol ferrous chloride and 6.25mg, 0.0168mmol of EDTA-2Na.2H 2 O), after stirring evenly, add 20g (0.1678mol) 2,3-dihydroindoline, stir, cool down to 10°C, add m-chloroperoxybenzoic acid in 5 batches, the total addition is 34g (0.1510mol, content 85%), after the addition is completed, keep warm at 10°C for 2 hours, and monitor the reaction by liquid chromatography. After the reaction is completed, heat and evaporate to remove the solvent, then add 60g of water, heat to reflux temperature, then cool to -5°C to crystallize, and After incubating at 5°C for 1 h, the crystals were collected by filtration and dried to obtain 19.58 g of the crystallized product 3-indoline, the content of which was detected to be 98.2%, and the yield was 97.8%. Concrete reaction formula is as follows:
[0039]
[0040] Wherein, by liquid phase analysis and detection, calculate the content of 3-hydroindole by e...
Embodiment 2
[0053] Synthesis of 2-methyl-3-indoline
[0054] Add 80g of anhydrous methanol and a catalyst (1.87g, 7.51mmol of copper sulfate pentahydrate and 0.87g, 7.51mmol of 1,2-bis(dimethylamino)ethane) into a 250mL four-neck flask, and stir evenly. Add 20g (0.1501mol) of 2-methylindoline, stir, and add di-tert-butyl peroxide dropwise at 25°C. The amount added is 21.08g. Track and monitor the reaction. After the reaction is completed, add 80g of water, heat to reflux temperature, then cool down to -4°C to crystallize, and keep it at -4°C for 0.5h, collect the crystals by filtration and dry to obtain the crystalline product 2-methyl- 19.60 g of 3-indoline, its content was detected to be 97.8%, and the yield was 97.3% (wherein, the calculation method of yield and content is the same as that of Example 1). Concrete reaction formula is as follows:
[0055]
[0056] Carry out to product 2-methyl-3-indoline 1 HNMR and LC-MS analysis, the characterization data obtained are as follows: ...
Embodiment 3
[0060] Synthesis of 3-Methyl-3,4-Dihydroquinoline
[0061] Add 80g of 2-propanol and 1.02g (5.44mmol) of copper nitrate into a 250mL four-neck flask, stir well, then add 20g (0.1359mol) of 3-methyl-1,2,3,4-tetrahydroquinoline, Stir, heat up to 40°C, add tert-butyl peroxybenzoate dropwise, the amount of addition is 25.86g, after the dropwise addition is completed, keep warm at 40°C for 0.5h, monitor the reaction with liquid chromatography, after the reaction is completed, heat and evaporate to remove the solvent, Then add 100g of water, heat to reflux temperature, then cool down to -6°C to crystallize, and keep it at -6°C for 1h, collect the crystals by filtration and dry to obtain the crystalline product 3-methyl-3,4-dihydroquinoline 19.63 g, the detected content is 97.8%, and the yield is 97.3% (wherein, the calculation method of yield and content is the same as that of Example 1). Concrete reaction formula is as follows:
[0062]
[0063] Carry out to product 3-methyl-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com