Cu-N-C oxygen reduction catalyst and preparation method thereof
A catalyst, cu-n-c technology, which is applied in electrical components, battery electrodes, circuits, etc., can solve the problems that the product is difficult to form exposed Cu-N active sites, difficult to control the electronic structure of active centers, and large copper ion diffusion coefficient. , to achieve the effect of promoting electron transfer, promoting exposure, and promoting mass transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This embodiment provides a method for preparing a Cu-N-C oxygen reduction catalyst, comprising the following steps:
[0045] S1), dissolving 1.0g of 1,10-phenanthroline in 100mL of absolute ethanol, stirring to form a transparent and uniform solution, then adding 0.50g of copper chloride dihydrate, and continuing to stir to form a complex precursor;
[0046] S2), slowly add 5g of nano-MgO with a size of 20nm to the solution obtained in step (1), ultrasonic for 15min, after the ultrasonic is completed, stir the solution at 60°C until the absolute ethanol is completely volatilized, then put it into a vacuum drying oven at 60°C Under drying for 12h, then the product was ground into powder;
[0047] S3), the powder porcelain boat in the step S2 is sent into and placed in the tube furnace, under N 2 Or in an Ar atmosphere, the temperature was raised to 900°C at a heating rate of 2°C / min for the first high-temperature heat treatment, and after holding for 2 hours, the temper...
Embodiment 2
[0051] This embodiment provides a method for preparing a Cu-N-C oxygen reduction catalyst, comprising the following steps:
[0052] S1), dissolving 0.66g of 1,10-phenanthroline in 100mL of absolute ethanol, stirring to form a transparent and uniform solution, then adding 0.20g of copper chloride dihydrate, and continuing to stir to form a complex precursor;
[0053] S2), slowly add 2.5g of nano-MgO with a size of 20nm to the solution obtained in step S1), ultrasonic for 15min, after the ultrasonic is completed, stir the solution at 60°C until the absolute ethanol is completely volatilized, then put it into a vacuum drying oven at 60°C Under drying for 12h, then the product was ground into powder;
[0054] S3), the powder porcelain boat in the step (2) is sent into and placed in the tube furnace, under N 2 Or in an Ar atmosphere, the temperature was raised to 800°C at a heating rate of 5°C / min for the first high-temperature heat treatment, and after holding for 2 hours, the te...
Embodiment 3
[0058] Performance Testing
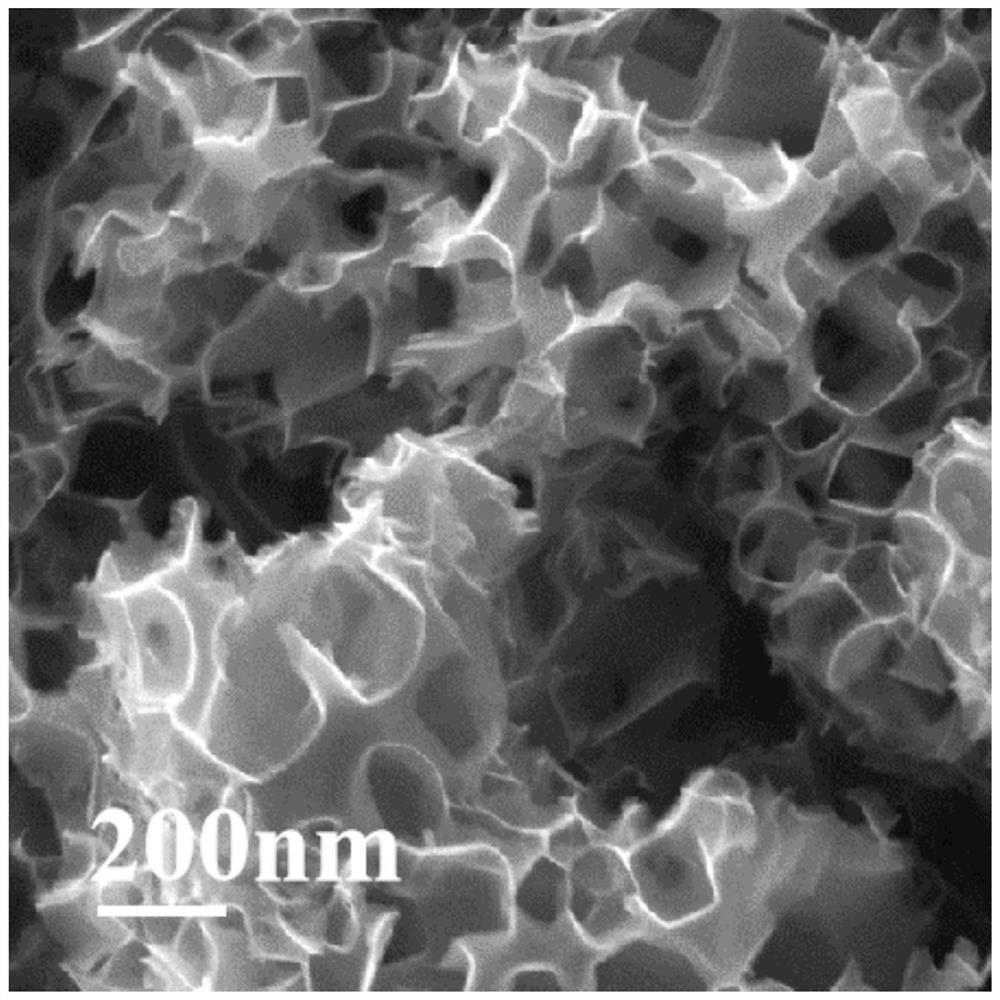
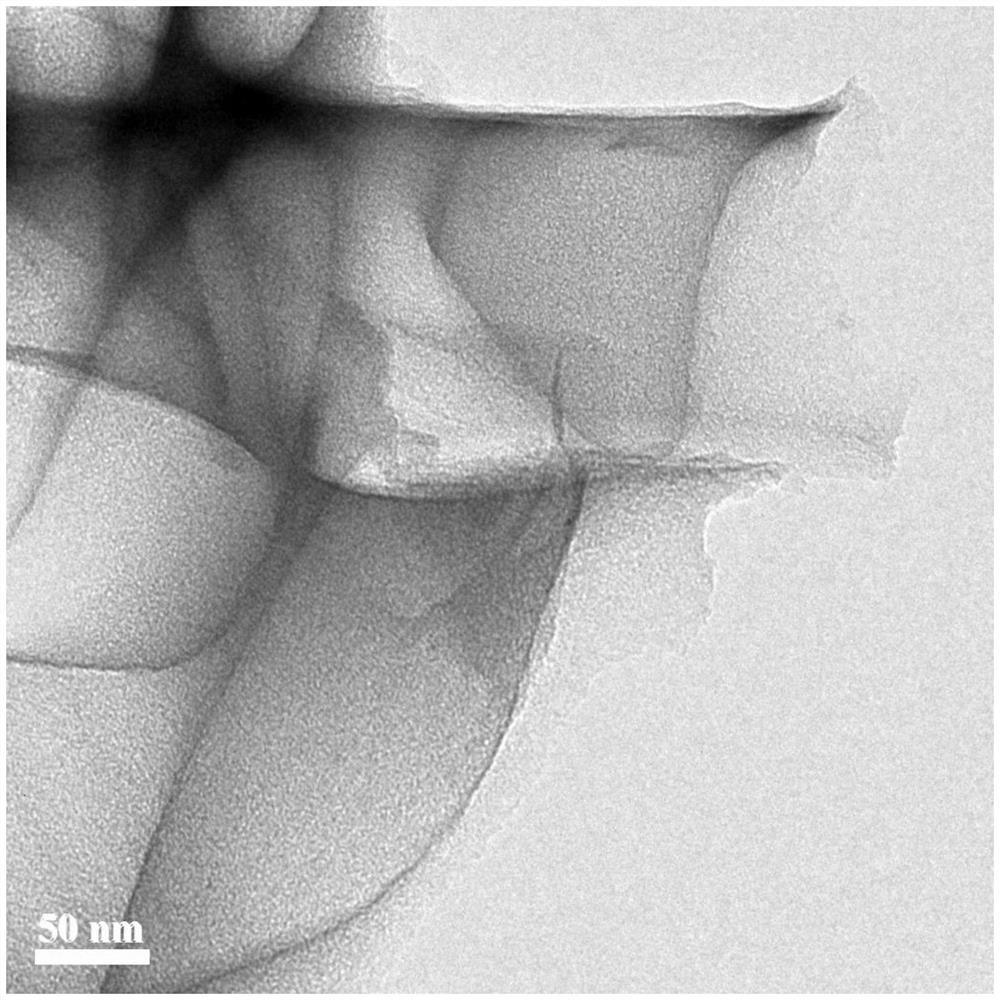
[0059] This embodiment takes the Cu-N-C catalyst prepared in Example 1 as the research object, and the structural characterization of the Cu-N-C catalyst is as follows Figure 1-5 As shown, the test results of its oxygen reduction performance as a catalyst are as follows Figure 6~7 Shown:
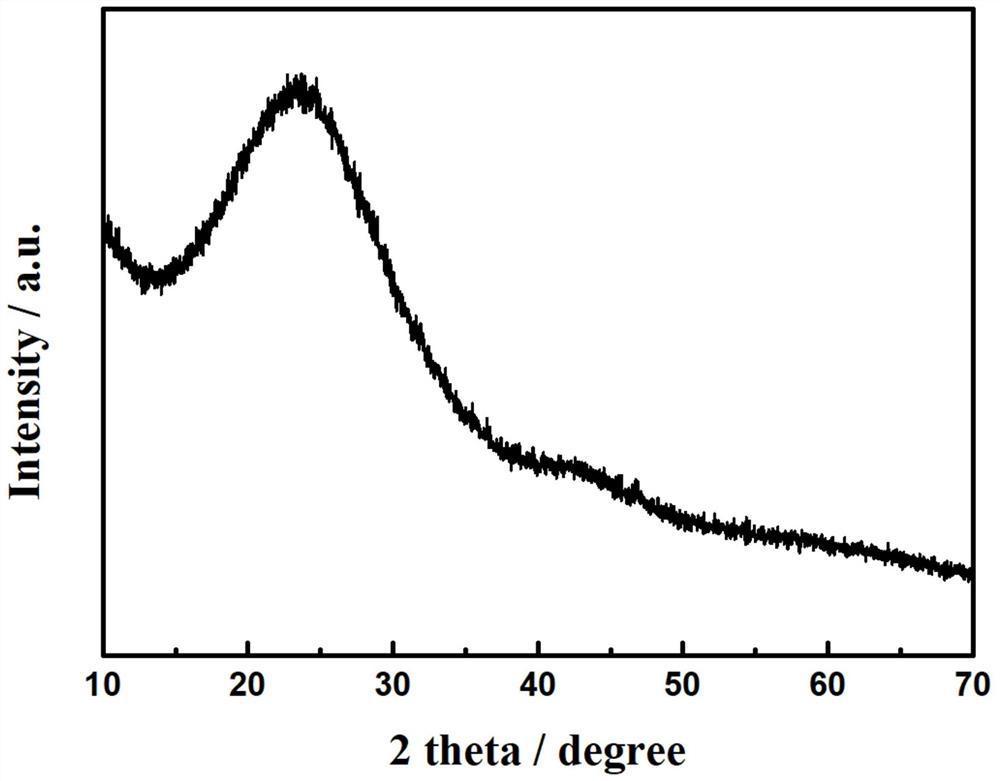
[0060] from figure 1 It can be observed that the two diffraction peaks at 2θ at 26.5 and 43.5° belong to the (002) and (101) crystal planes of graphitized carbon, respectively, which proves that the transition metal in the precursor can effectively catalyze the precursor to form graphitized carbon Material. And there is no diffraction peak related to Cu compounds (such as Cu 0 、Cu 2 O or CuO, etc.), indicating that the MgO template method used in the present invention can spatially isolate Cu ions in the complex precursor, thereby forming a highly dispersed Cu-N-C catalyst without metal agglomeration during heat treatment.
[0061] from figure 2 It can ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com