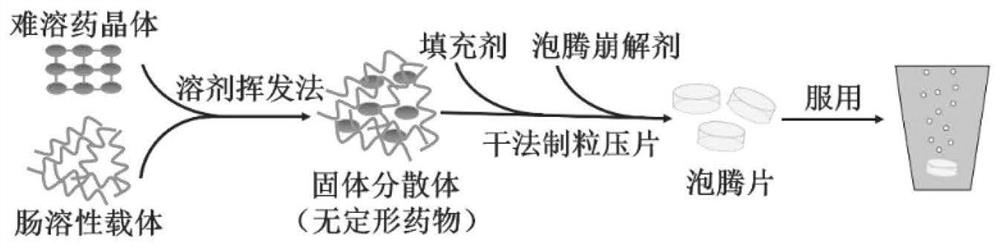
Effervescent tablets containing stiripentol solid dispersion and preparation method thereof
A technology of solid dispersion and stiripentol, which is applied to medical preparations containing active ingredients, dispersion liquid delivery, and pharmaceutical formulations, etc. It can solve problems such as poor material safety, reduced drug permeability, and poor patient compliance , to achieve the effect of improving intestinal permeability, improving solubility, and taking convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: Recipe optimization and solid-state characterization of solid dispersions
[0078] The preparation process is as figure 1 As shown, the formulation optimization and solid-state characterization are as follows:
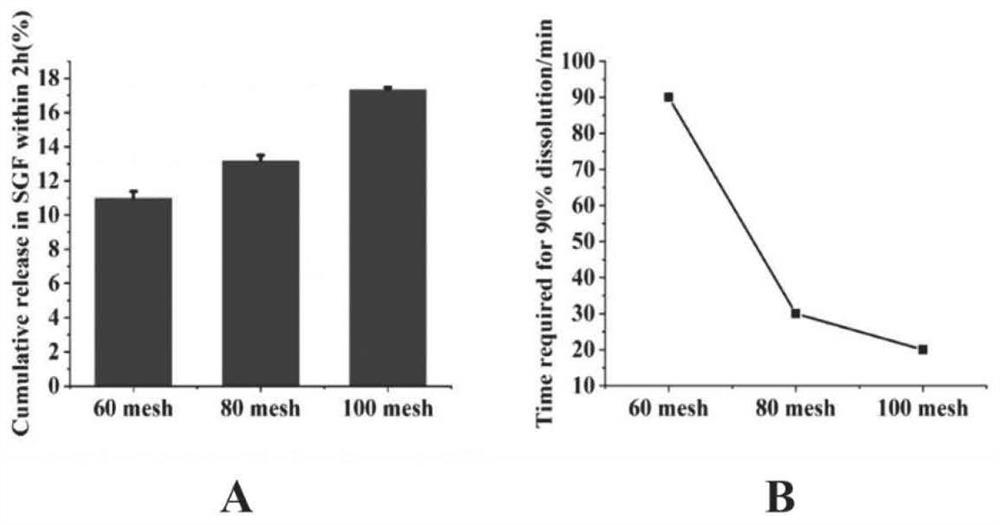
[0079] 1) Effect of sieve mesh on dissolution behavior of solid dispersion in gastric juice and intestinal juice
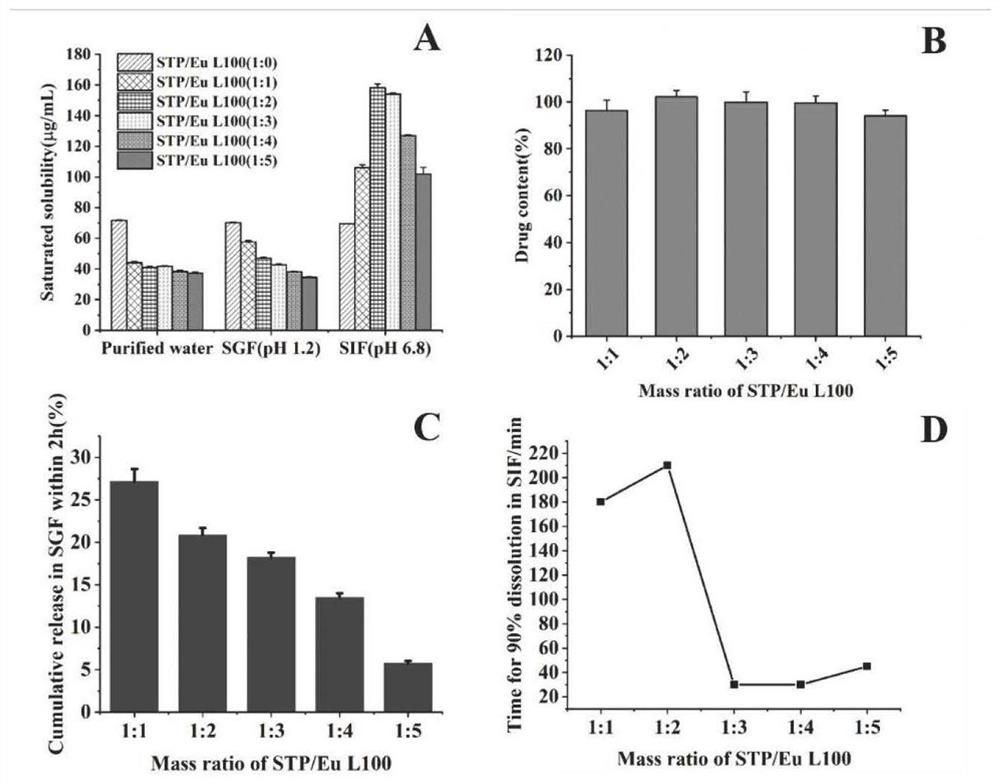
[0080] Accurately weigh 100mg of stiripentol and 400mg of Eudragit L100, dissolve them in 3mL of mixed solvent (ethanol: dichloromethane = 1:1, v / v) successively, dissolve them completely by ultrasonication in a water bath, and then vortex for 2 minute. Organic solvents were removed under vacuum on a rotary evaporator at 45°C, 180 rpm. The obtained dried solid system was stored in a desiccator at room temperature for 24 hours before crushing and sieving, and then it was taken out and pulverized into coarse particles and passed through 60, 80 and 100 mesh screens respectively to obtain solid dispersions of different sizes. For dissolut...
Embodiment 2
[0100] Embodiment 2: the preparation method and prescription screening of effervescent tablet
[0101] 1) Optimization of effervescent tablet preparation method
[0102] For powder direct compression method, at first all auxiliary materials are all passed through 80 mesh sieves respectively, then the anhydrous citric acid of prescription quantity is mixed with direct compression type mannitol for 10 minutes to obtain mixture 1, then the sodium bicarbonate of prescription quantity and The solid dispersion was mixed for 10 minutes to obtain mixture 2, and finally the mixtures 1 and 2 were fully mixed and then compressed into tablets immediately. For dry granulation and tabletting, the flakes obtained by the above-mentioned powder direct compression method are crushed into coarse particles, passed through a 20-mesh sieve for sizing, and immediately pressed into tablets according to the prescribed amount. For wet granulation tableting, first pass all excipients through 80-mesh si...
Embodiment 3
[0116] Embodiment 3: In vivo intestinal perfusion experiment studies the penetration enhancement effect of stiripentol solid dispersion
[0117] 1) Physiological saline: Accurately weigh 9g of NaCl and dissolve it in 1000mL of water.
[0118] 2) Chloral hydrate injection: accurately weigh 3.3 g of chloral hydrate, and dilute to 100 mL with normal saline.
[0119] 3) Krebs-Ringer buffer: 7.8g NaCl, 0.35g KCl, 0.37g CaCl per 1000mL water 2 ,0.22gMgCl 2 ,0.22g NaH 2 PO4, 1.4g C 6 h 12 o 6 ,1.37g NaHCO 3 , adjust the pH of the buffer to 7.4 with phosphoric acid or sodium hydroxide.
[0120] 4) Intestinal perfusate: Accurately weigh an appropriate amount of STP solid dispersion, and use pH 7.4 Krebs-Ringer buffer to prepare a concentration of 100 μg·mL -1 The intestinal perfusate was ultrasonically dissolved for use as the test solution. Accurately weighed 20 mg of STP raw material, and prepared 200 mL of STP suspension solution with pH 7.4 Krebs-Ringer buffer as a control...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com