Fluorescent probe for use in detection of brain tumor
A fluorescent probe and brain tumor technology, applied in the field of fluorescent probes, can solve the problems of inability to carry out fluorescent labeling, duration, difficulty in re-administration, and difficulty in improving brain nerve function preservation while taking into account the resection rate, and achieves excellent practicability. , Improve the removal rate, the effect of simple detection steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] 1. Synthesis of fluorescent probes
[0095]Hydroxymethylrhodamine green (HRMG) with various dipeptide sites was synthesized according to the reaction scheme described in Example 1 of International Publication WO2016 / 006678. Compounds with different dipeptide sites were obtained by making various changes to the Fmoc-amino acid used in the synthesis of compound A7 in Example 1.
[0096] In the synthesized compound, R in the above general formula (I) 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 and R 9 Both are hydrogen atoms, X=O, Y=methylene, and P1 and P2 are the following combinations.
[0097] Table 1
[0098] compound P1 P2 YK190 Arginine residues proline YK213 Histidine Glycine YK19 Tyrosine Glycine YK281 methionine alum Acetylleucine
Embodiment 2
[0100] 2. Screening by fluorescent probes
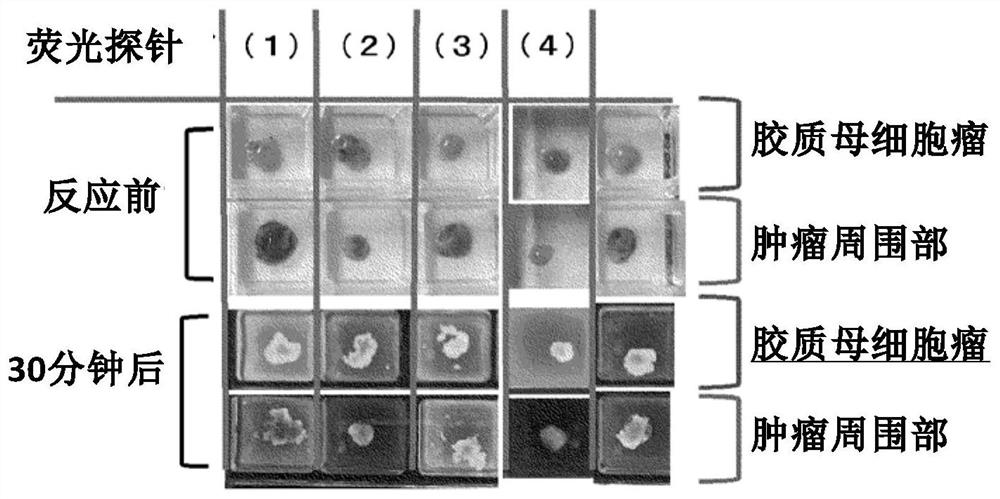
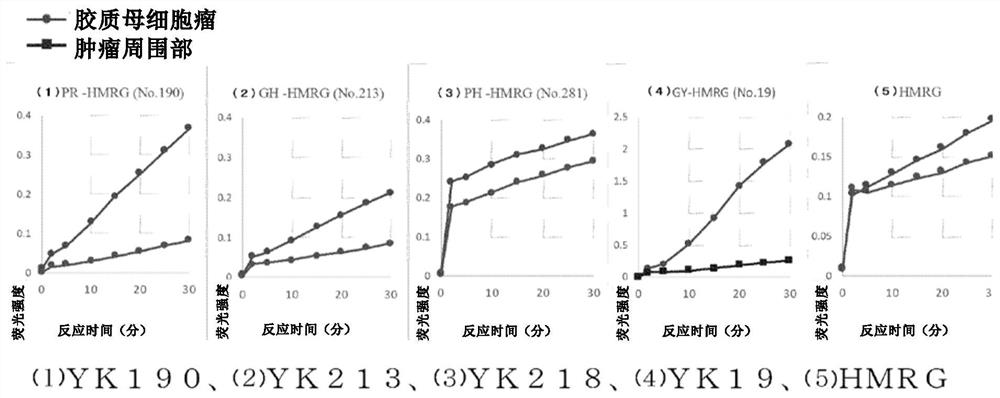
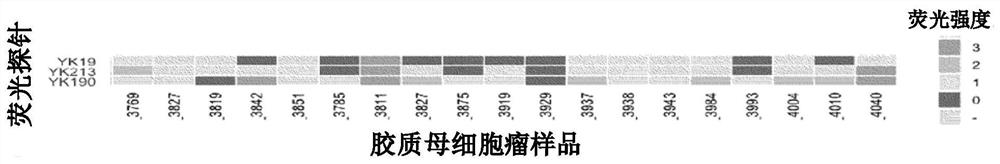
[0101] The fluorescent probe compound group containing YK190, YK213, YK19 and YK281 synthesized in Example 1 was added to the fresh samples of the tumor and the surrounding part of the tumor, and the fluorescence intensity was compared. The sample used was glioblastoma. As a comparative example, hydroxymethylrhodamine green (HRMG) without a dipeptide site was used.
[0102] Dissolve 0.5 μL DDMSO solution (10 mM) of various fluorescent probes in 100 μL of RPMI1640 (phenol red-free) (the final concentration of the probe is 50 μM), drop 200 μL to each sample, and use Maestro InVivo Imaging The System Ex measures fluorescence intensity over time.
[0103] Excitation wavelength: 465nm
[0104] ·Emission wavelength: 515nm
[0105] Such as figure 1 and figure 2 As shown in the results, it was found that (1) YK190 and (2) YK213 were tumor-specific fluorescently labeled compared with the peripheral tissue, while (3) YK218 was not much ...
Embodiment 3
[0108] 3. Protease Identification
[0109] Next, proteases associated with the fluorescent response obtained in Example 2 were identified.
[0110] Cathepsin D, cytosolic non-specific dipeptidase, calpain 1, cytosolic aminopeptidase were identified using DEG (Diced electrophoresis gel) assay and LC MS / MS by peptide mass fingerprinting for protease analysis as candidate enzymes.
[0111] For these candidate enzymes, as a result of measuring the changes in fluorescence intensity using inhibitors (SNJ-1945 and Amastatin), it was found that the fluorescence of calpain 1 and cathepsin D was inhibited by SNJ-1945.
[0112] For these calpain 1 and cathepsin D, when measuring the change in fluorescence intensity due to the reaction with the fluorescent probe YK190, as shown in 4, a significant increase in fluorescence intensity was observed, indicating that the fluorescence of glioblastoma in Example 2 The response is caused by the reaction of these enzymes with fluorescent probes. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com