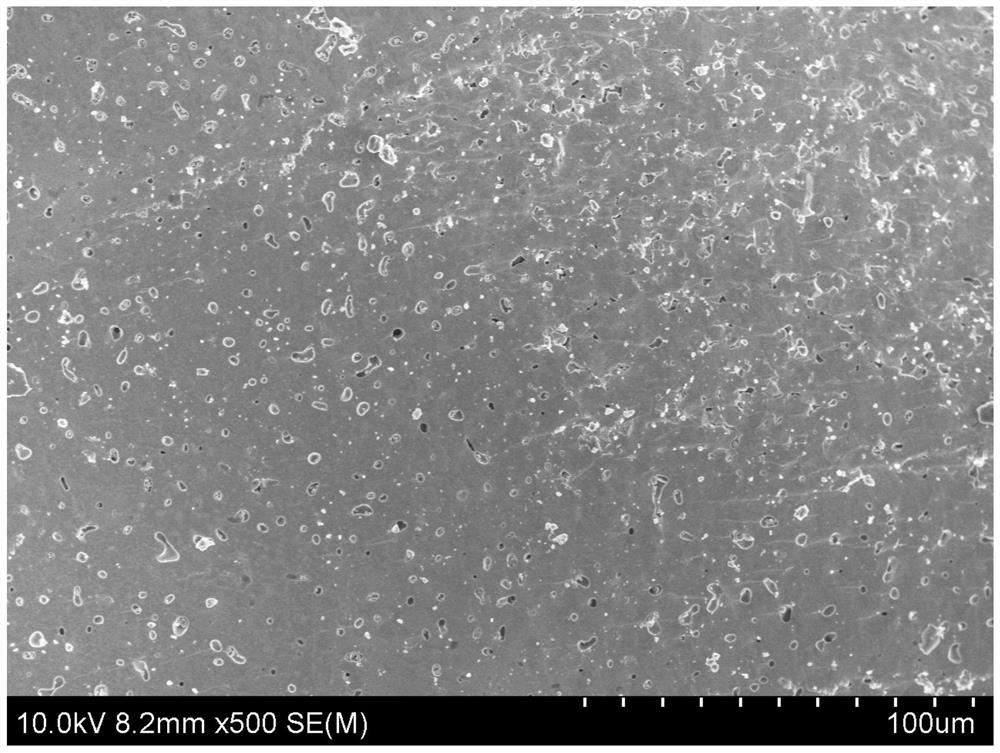
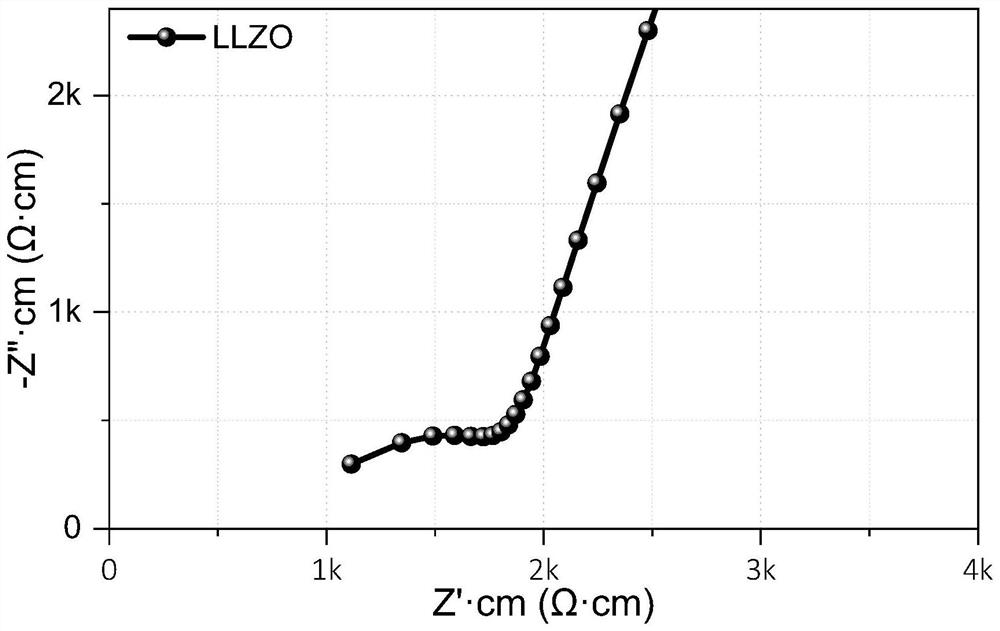
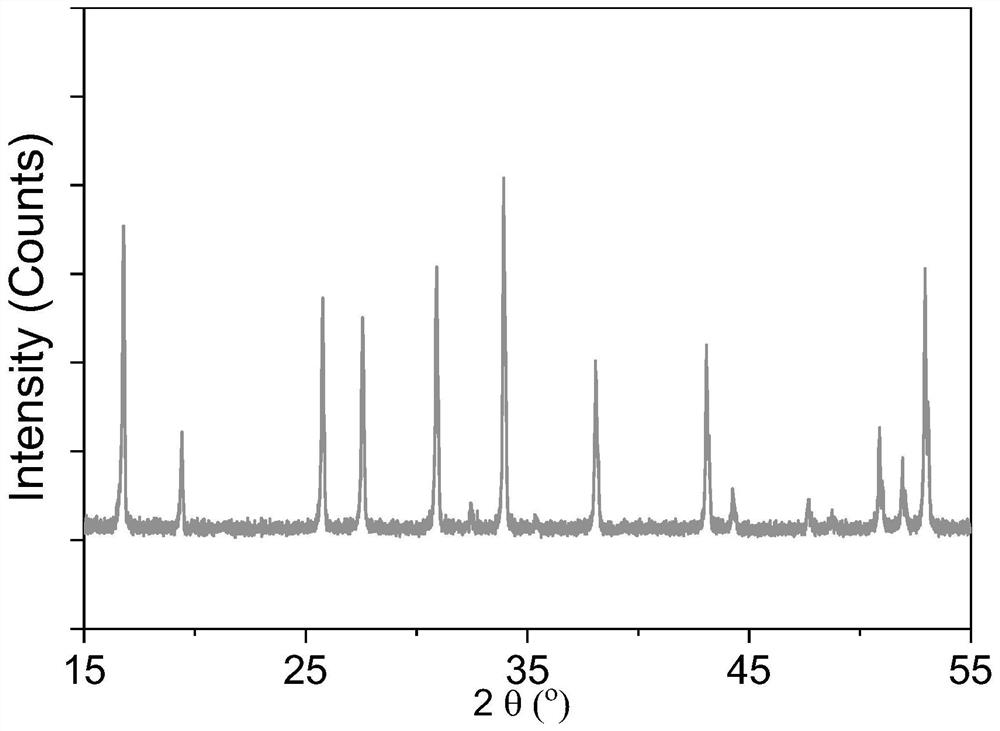
Dry preparation process of solid electrolyte
A technology for solid electrolyte and preparation process, applied in the field of dry preparation process of solid electrolyte, can solve the problems of volatilization and loss of lithium, and achieve the effects of reducing volatilization of lithium, avoiding pollution and harm, and high density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Weigh lithium hydroxide, lanthanum oxide, zirconium oxide, and tantalum oxide sequentially according to the mass fractions of 15%, 50%, 20%, and 15%, respectively, using zirconia balls and polyurethane ball milling tanks, and dry method at a speed of 175r / min Ball mill for 2 hours, then use a sieve to separate the balls and powder. Transfer the powder to an alumina crucible for pre-sintering. The sintering system is 5°C / min and the temperature is raised to 950°C. After 3 hours of heat preservation, it is cooled to room temperature with the furnace, and the secondary dry ball milling is performed at a speed of 175r / min. Zirconium balls and polyurethane ball milling tank, the ball milling time is 2 hours, after ball milling, the ball material is separated to obtain master powder. Weigh 1 g of mother powder, hold the pressure at 500 MPa for 1 min to obtain a green body, the diameter of the green body is 12 mm. The ceramic green body was transferred to a magnesia crucible w...
Embodiment 2
[0040] Weigh lithium hydroxide, lanthanum oxide, zirconium oxide, and tantalum oxide sequentially according to the mass fractions of 30%, 45%, 15%, and 10%, respectively, using zirconia balls and polyurethane ball milling jars, and dry method at a speed of 300r / min Ball milling was carried out for 0.5 hour, after which the balls and powder were separated using a screen. Transfer the powder to an alumina crucible for pre-sintering. The sintering system is 5°C / min and the temperature is raised to 900°C. After 3 hours of heat preservation, it is cooled to room temperature with the furnace, and the secondary dry ball milling is performed at a speed of 300r / min. Zirconium balls and polyurethane ball milling tank, the ball milling time is 0.5 hours, after ball milling, the ball material is separated to obtain the mother powder. Weigh 0.5g of mother powder, hold the pressure at 100MPa for 1min to obtain a green body, the diameter of the green body is 12mm. The ceramic green body was...
Embodiment 3
[0043] Weigh lithium hydroxide, lanthanum oxide, zirconium oxide, and tantalum oxide sequentially according to the mass fractions of 15%, 50%, 20%, and 15%, using tungsten carbide balls and nylon ball mill jars, and dry method at a speed of 250r / min Ball mill for 2.5 hours, then use a screen to separate the balls and powder. Transfer the powder to an alumina crucible for pre-sintering. The sintering system is 5°C / min to 850°C. After 6 hours of heat preservation, it is cooled to room temperature with the furnace. The secondary dry ball milling is carried out at a speed of 175r / min. Tungsten ball and nylon ball milling tank, the ball milling time is 2 hours, after ball milling, the ball material is separated to obtain the mother powder. Weigh 2g of mother powder, hold the pressure at 1000MPa for 1min to obtain green body, the diameter of green body is 12mm. The ceramic green body was transferred to a magnesia crucible with an inner diameter of 15mm, and the secondary sintering ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com