Zeranol immunoaffinity column as well as preparation method and application thereof
A technology of zearalanol and immunoaffinity, applied in the field of immunoaffinity chromatography, can solve the problems of inability to selectively adsorb target molecules, introduce interference substances, cumbersome operation, etc., simplify the sample pretreatment process and improve the analysis quality , good purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 zearalenol monoclonal antibody
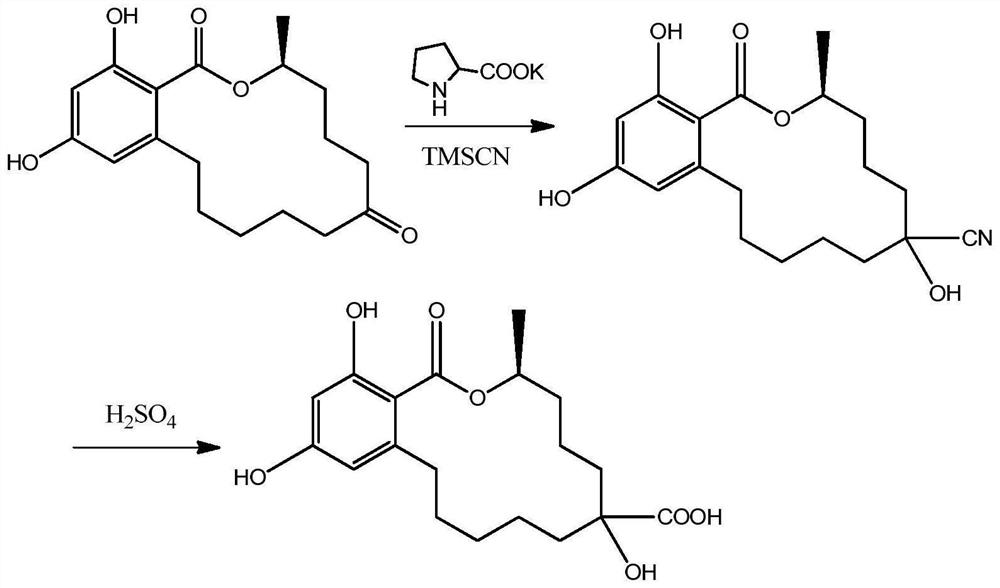
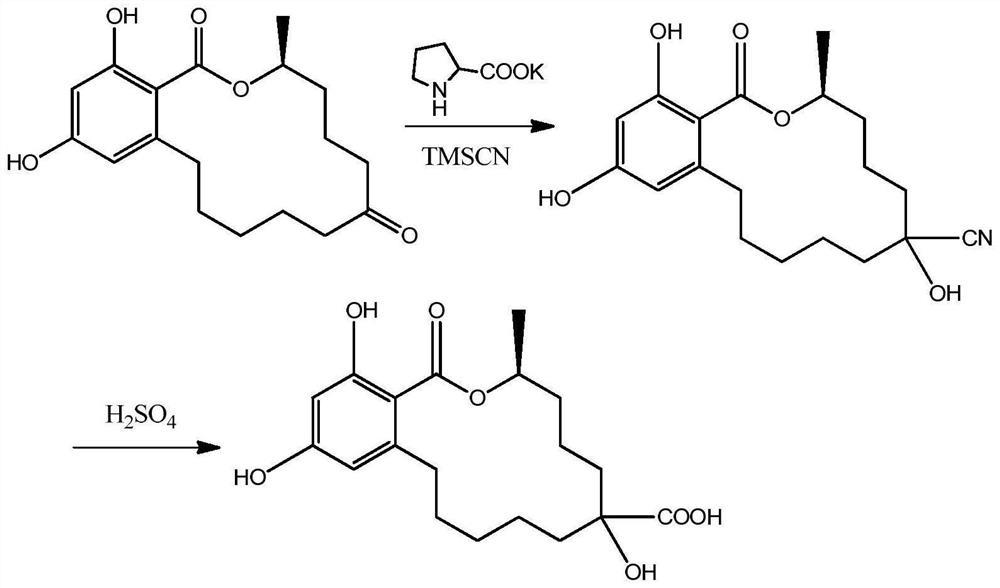
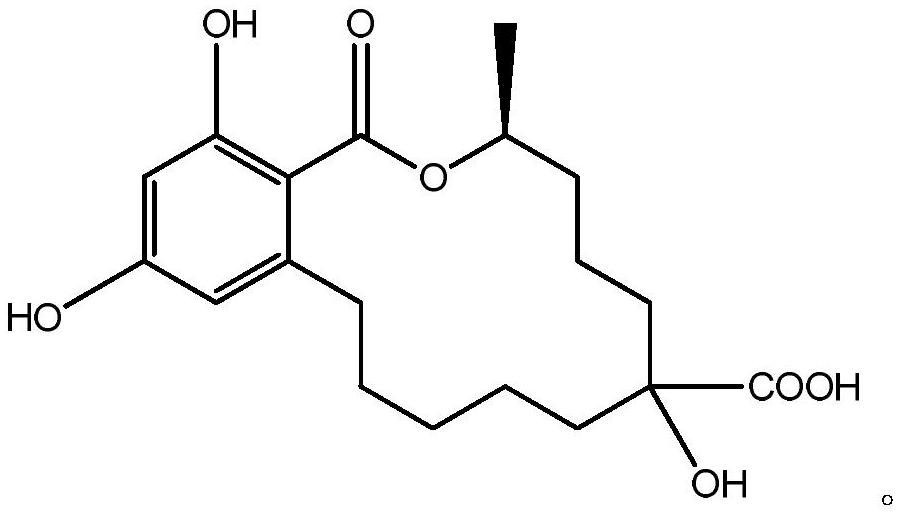
[0021] 1. Synthesis of zearalenol hapten (synthetic route see attached figure 1 )
[0022] Take 0.32g of zearalanone, add 10mL of N,N-dimethylformamide (DMF) to dissolve, add 0.13g of potassium L-hydroxyproline, stir well, add 2.12g of trimethylsilyl cyanide, and stir at room temperature for 8h , stop the reaction, add water 20mL, 1mol / L hydrochloric acid 3mL, fully stir for 30min, stop stirring, add water 50mL, add ethyl acetate 20mL×3 for extraction three times, combine the organic phases, evaporate to dryness to obtain a red oil; Dissolve in 80% sulfuric acid, react at 90°C for 4 hours, stop the reaction, add 6mol / L NaOH to adjust the pH value to 6, add 100mL of dichloromethane for extraction, separate the water phase, evaporate the organic phase to dryness, put on a silica gel column, and use The mixed solution of dichloromethane and methanol with a volume ratio of 10:1 was eluted and separated to ob...
Embodiment 2
[0039] Example 2 Preparation of Zearallol Immunoaffinity Column
[0040] 1. Activation of solid phase carrier
[0041] Weigh 1 g of cyanogen bromide-activated agarose gel 4B, add 10 mL of 1 mmol / L HCl solution, soak for 30 min to make it swell; then centrifuge at 3000-8000 r / min for 2-10 min to separate the solid-phase carrier, Wash the swollen solid-phase carrier with pH 9.0 CB buffer 3 to 5 times, adding 10 mL of buffer solution each time, and finally centrifuge at 5000 r / min for 10 min to separate the solid-phase carrier.
[0042] 2. Antibody Conjugation
[0043] Add 30mg of zearalenol monoclonal antibody to the activated solid phase carrier, then add 10mL of 0.1mol / L pH9.0 CB buffer solution, and shake at 150r / min at 37°C for 1h; after the reaction, centrifuge Conjugates, with 0.1mol / L NaHCO pH 8.3 3 The conjugate was washed with buffer for 3 to 5 times, and 10 mL of buffer was added each time.
[0044] 3. Antibody blocking
[0045] Add 10 mL of 0.1 mol / L Tris-HCl solut...
Embodiment 3
[0048] The use of embodiment 3 zearalenol immunoaffinity columns
[0049] 1. Sample preparation
[0050] Accurately weigh 10.0g (accurate to 0.01g) homogeneous sample, add 2.0g sodium chloride; mix with 40.0mL methanol-water solution (80:20, V / V), extract by shaking for 15min, and centrifuge at 4000r / min 5min; accurately pipette 10mL supernatant, add 40mL PBS solution to dilute, mix well, filter with glass microfiber filter paper until the filtrate is clear, and set aside.
[0051] 2. Immunoaffinity column purification
[0052] Accurately pipette 10mL of the above-mentioned filtrate, inject it into a syringe, adjust the pressure of the syringe to make it slowly pass through the immunoaffinity column at a speed of 2 s / drop; wash the column with 20mL water (for the sample solution with a darker color, first use 10mL 0.1% spit Wash with warm 20-PBS, then wash with 10mL 10% methanol-water, and finally wash with 10mL water), flow rate 1s / drop, discard the effluent until 2-3mL air...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com