GH11 family xylanase gene, cloning expression and ramie degumming application thereof
A technology for xylanase and xylan, which is applied in the field of genetic engineering, can solve the problems of poor pH tolerance and low catalytic efficiency of xylanase, and achieves the improvement of both enzyme activities, strong adaptability of metal ions, and significant improvement. The effect of industrial application advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Gene Cloning of Aspergillus terreus-derived xylanase xyl-1, xyl-2
[0059] Filter the mycelium cultivated for 3-4 days with sterile filter paper and put it into a mortar, add liquid nitrogen to grind, add the extract at the same time, then add 0.2ml chloroform, and centrifuge at 12000rpm for 15min after ice bathing for 10min. Finally, add the centrifuged supernatant to the extraction tube, add RNA wash buffer 1 and 2 respectively for centrifugation, and finally dissolve it in DPEC water, centrifuge to recover the liquid, and store it in a -20°C refrigerator for later use.
[0060] Thermally denature the extracted RNA for 5 minutes, add reaction solution to remove genomic DNA, and perform reverse transcription PCR reaction (reaction at 37°C for 15 minutes, reaction at 50°C for 5 minutes, reaction at 98°C for 5 minutes) to obtain the cDNA sequence, and store at -80°C .
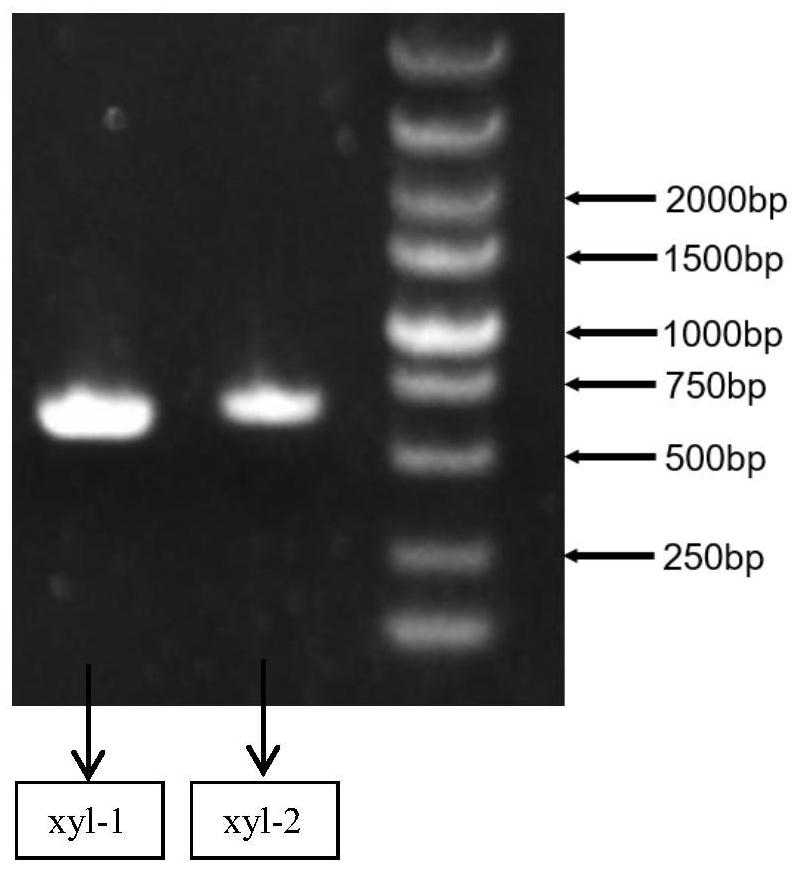
[0061] According to the genome of Aspergillus terreus NIH2624, 2 primers for xylanase xyl-...
Embodiment 2
[0064] Example 2: Heterologous expression of recombinant xylanase xyl-1 and xyl-2
[0065] Inoculate recombinant strains E.coli BL21 / xyl-1 and E.coli BL21 / xyl-2 into 100ml liquid LB culture based on 37°C and 180rpm culture, when OD 6oo When it reaches about 0.6-0.8, add IPTG to induce the expression of the enzyme. Then transfer to 16°C and 100rpm to cultivate for 16h, then centrifuge at 10000rpm for 10min to collect the bacteria. Suspend the cells with an appropriate amount of PBS buffer solution with a pH of 7.0, break the cells with an ultrasonic cell disruptor in an ice bath, centrifuge at 4°C and 8000 rpm / min for 10 min, and the supernatant is the crude enzyme solution.
Embodiment 3
[0066] Embodiment 3: Purification of xylanase xyl-1, xyl-2
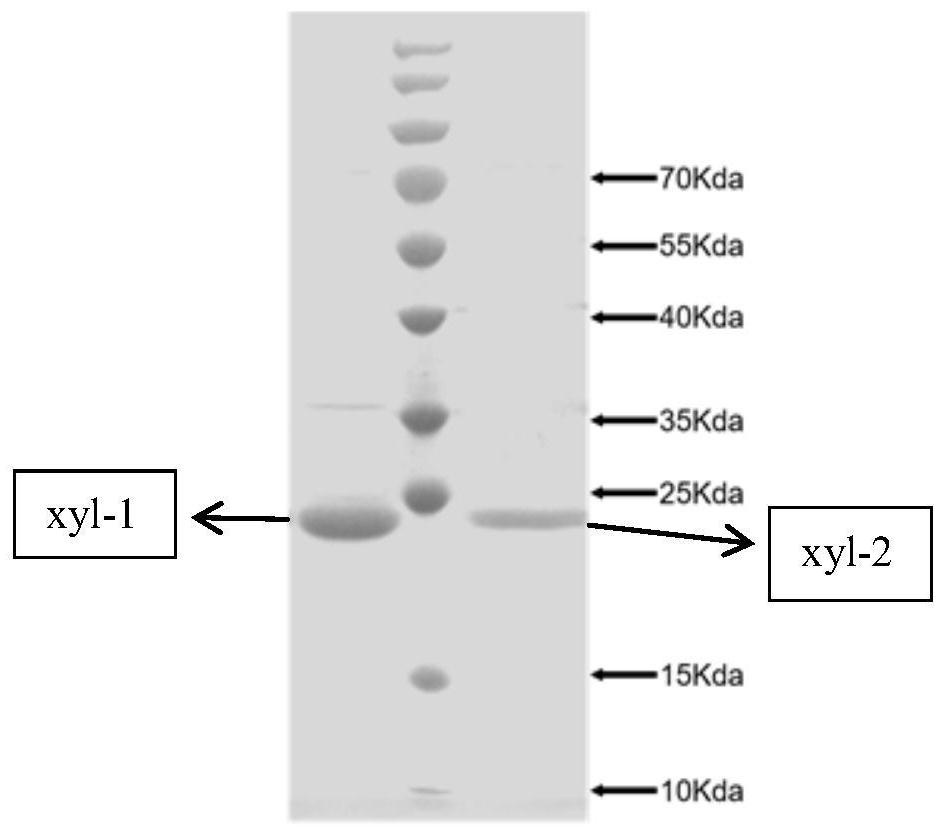
[0067] First equilibrate the column with 10 times the column volume Binding buffer, and then load the crude enzyme solution at a flow rate of 20 times the column volume / hour; wash the column with an eluent of 15 times the column volume (10mM imidazole) to wash away the impurity proteins; The target protein was eluted with 5 times column volume Elutionbuffer (200mM imidazole), and the eluate was collected; then the column was washed with 15 times column volume (500mM imidazole) eluent; finally, the purified protein was desalted using a desalting spin column.
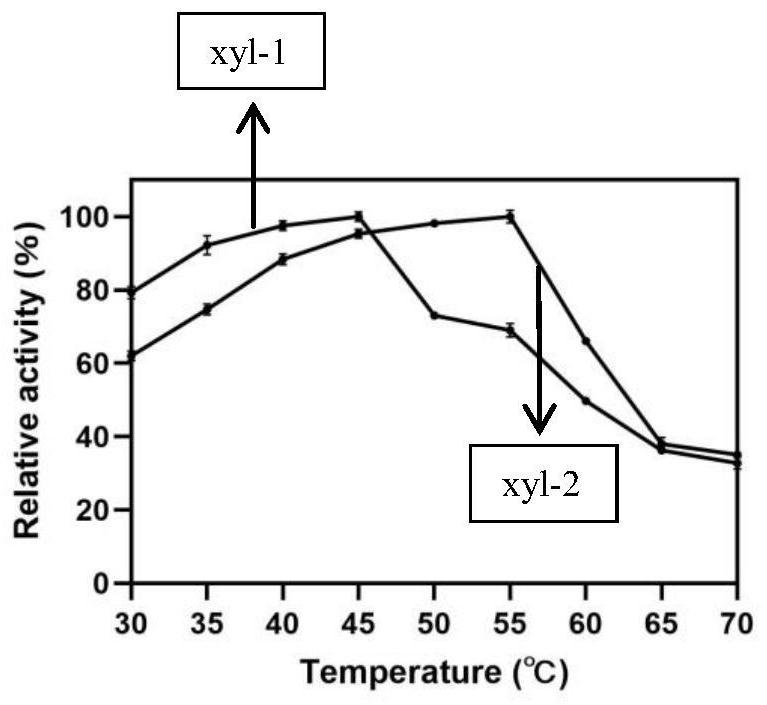
[0068] The collected enzyme solution was detected by SDS-PAGE, and the results were as follows: figure 2 shown. The protein concentration was determined with bovine serum albumin (BSA) as the standard.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com