Preparation method of self-supporting bifunctional water electrolysis catalyst
A self-supporting electrode, dual-function technology, applied in the field of catalysis, can solve problems such as energy shortage, achieve the effect of easy control, improve stability, and improve the problem of charge transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
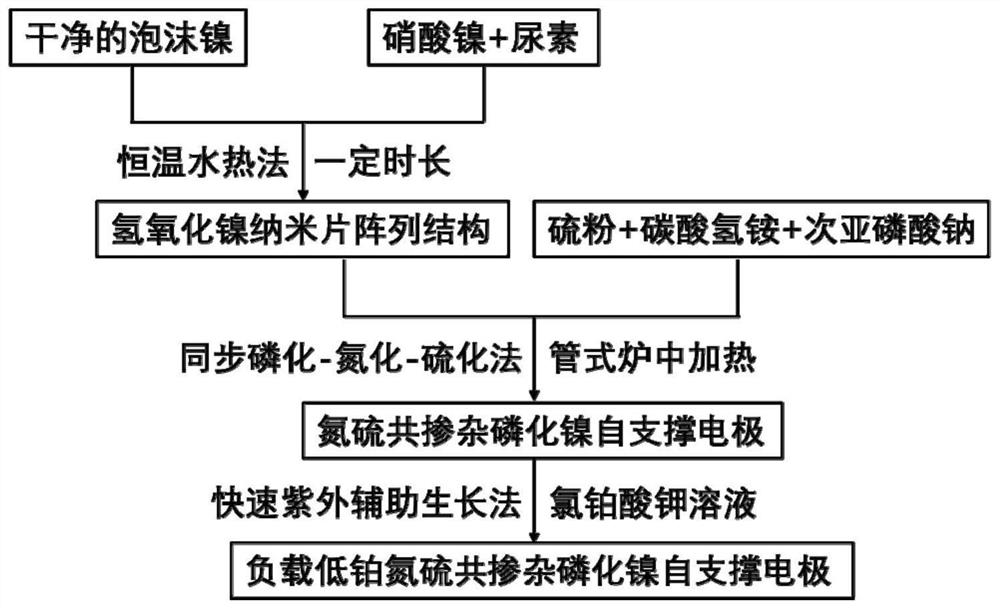
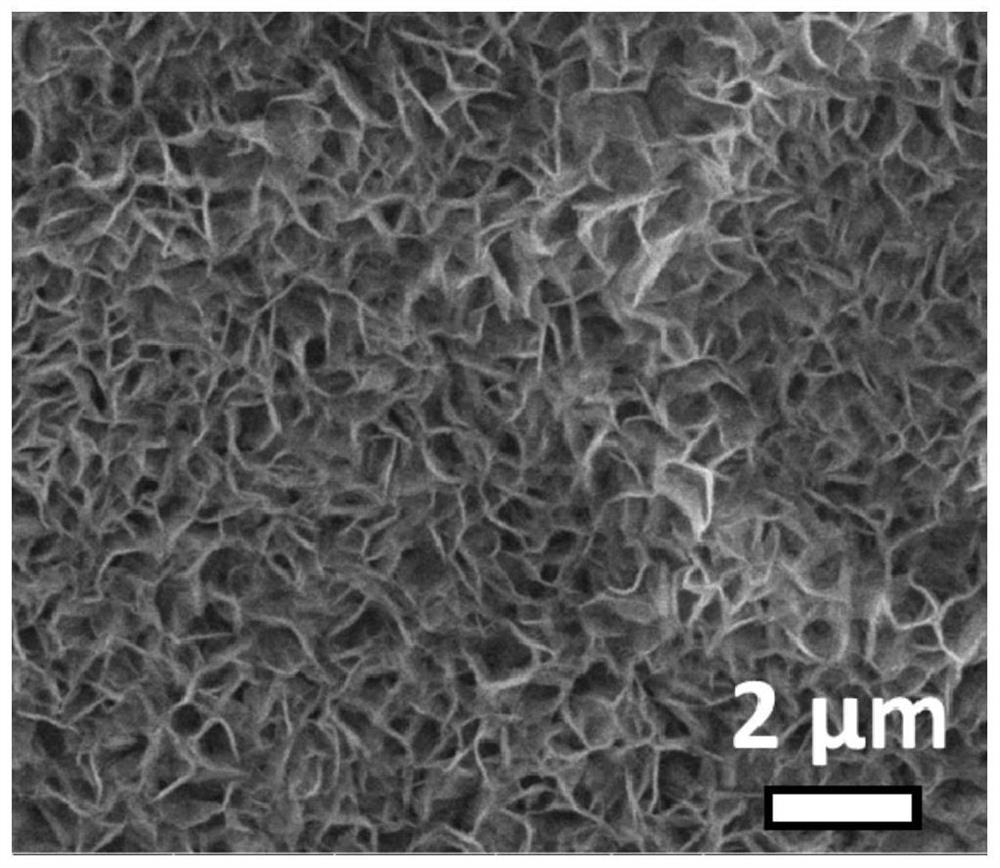
[0028] (1) Adopt the hydrothermal growth method, take clean nickel foam (1.0mm) as the substrate, weigh 0.5g of nickel nitrate hexahydrate, 0.6g of urea and disperse them in 30ml of deionized water, stir to dissolve evenly, and transfer them to the reaction kettle. After packaging the shell, transfer it to a constant temperature oven, keep it at 100°C for 8 hours, and then cool down naturally. The product was taken out from the reaction kettle, rinsed repeatedly with deionized water and ethanol, and dried in a vacuum oven to prepare a nickel hydroxide nanosheet array electrode.
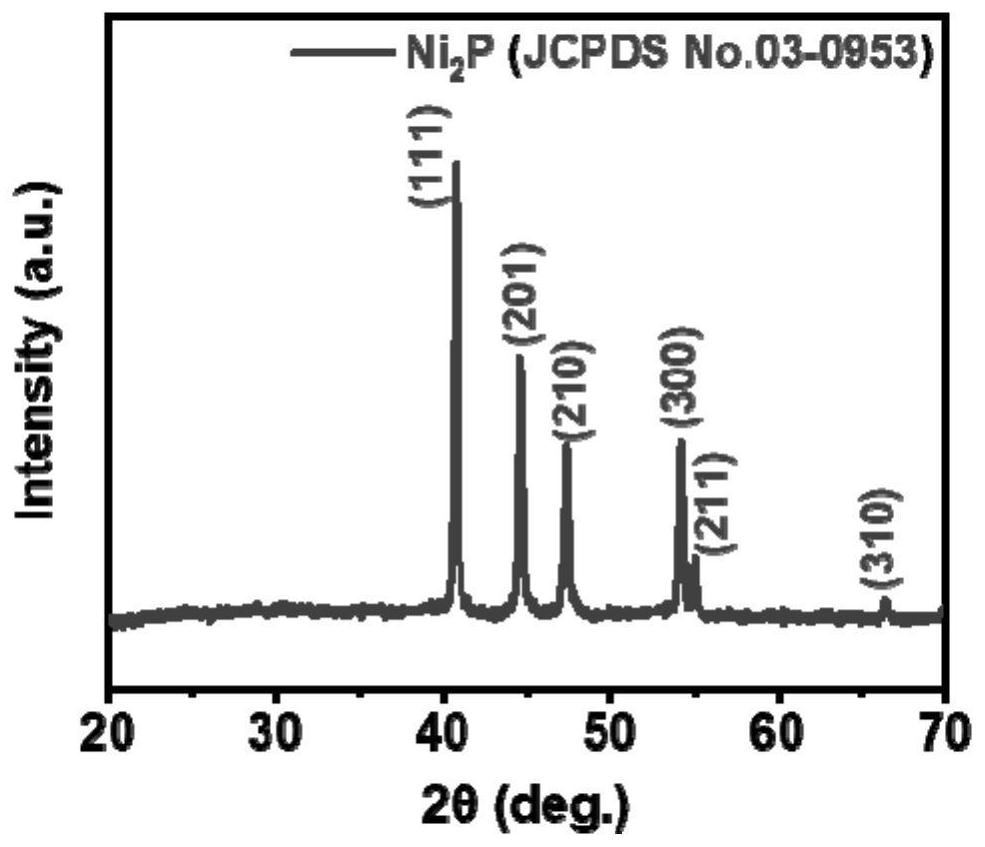
[0029] (2) Adopting the simultaneous phosphating-nitriding-sulfurization method, the cobalt hydroxide nanosheet array obtained in step (1) is placed downstream of the air outlet of the tube furnace, and then 0.05g of sulfur powder and 0.1g of ammonium bicarbonate are weighed respectively, Sodium hypophosphite solid 0.5g, and placed in the upper tuyeres of the tube furnace in turn, then nitrogen gas wa...
Embodiment 2
[0032] (1) Adopt the hydrothermal growth method, take clean nickel foam (1.5mm) as the substrate, weigh 0.6g of nickel nitrate hexahydrate, 0.7g of urea and disperse them in 35ml of deionized water, stir to dissolve evenly, and transfer them to the reaction kettle. After packaging the shell, transfer it to a constant temperature oven, keep it at 90°C for 10 hours, and then cool down naturally. The product was taken out from the reaction kettle, rinsed repeatedly with deionized water and ethanol, and dried in a vacuum oven to prepare a nickel hydroxide nanosheet array electrode.
[0033] (2) Adopting the simultaneous phosphating-nitriding-sulfurization method, the cobalt hydroxide nanosheet array obtained in step (1) is placed downstream of the air outlet of the tube furnace, and then 0.1g of sulfur powder and 0.15g of ammonium bicarbonate are weighed respectively, Sodium hypophosphite solid 1.0g, and placed in the upper tuyere of the tube furnace in turn, followed by argon gas...
Embodiment 3
[0036] (1) Adopt the hydrothermal growth method, take clean nickel foam (1.7mm) as the substrate, weigh 0.8g of nickel nitrate hexahydrate, 0.8g of urea and disperse them in 40ml of deionized water, stir to dissolve evenly, and transfer them to the reaction kettle. After packaging the shell, transfer it to a constant temperature oven, keep it at 110°C for 9 hours, and then cool down naturally. The product was taken out from the reaction kettle, rinsed repeatedly with deionized water and ethanol, and dried in a vacuum oven to prepare a nickel hydroxide nanosheet array electrode.
[0037] (2) Adopting the simultaneous phosphating-nitriding-sulfurization method, the cobalt hydroxide nanosheet array obtained in step (1) is placed downstream of the air outlet of the tube furnace, and then 0.15g of sulfur powder and 0.2g of ammonium bicarbonate are weighed respectively, Sodium hypophosphite solid 1.5g, and placed in the upper tuyere of the tube furnace in turn, then nitrogen gas was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com