Alkynyl-terminated polyphenyl ether as well as preparation method and application thereof
A polyphenylene ether and alkynyl technology, which is applied in the field of alkynyl-terminated polyphenylene ether compounds and their preparation, can solve the problem that it is difficult to ensure that the end-capped group does not undergo a curing reaction in advance, and the cross-linking density of double bonds and other resins Limited, low efficiency of end-capped polyphenylene ether, etc., to achieve the effect of excellent processability, low intrinsic viscosity, and reduction of process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
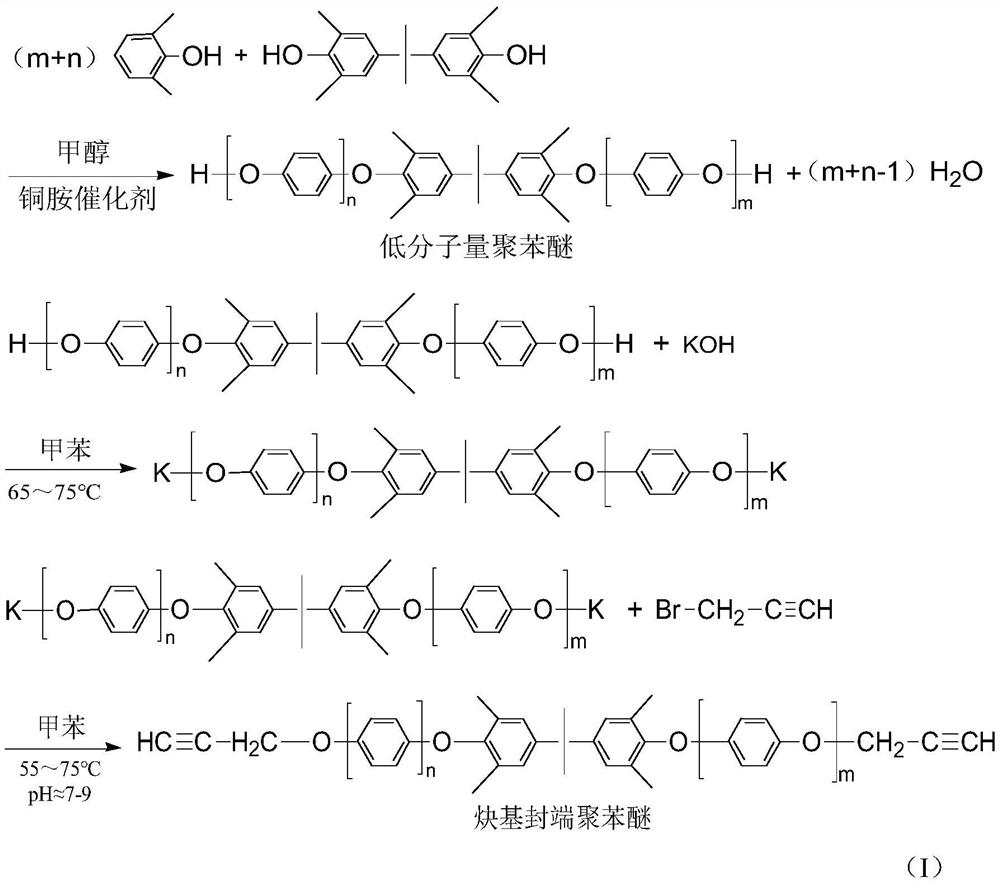
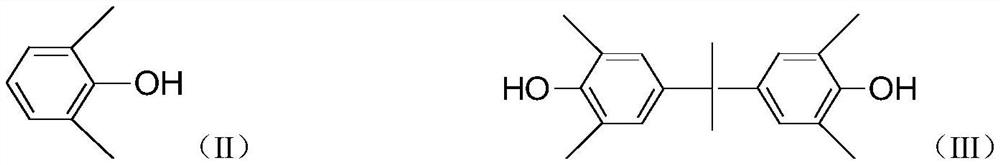
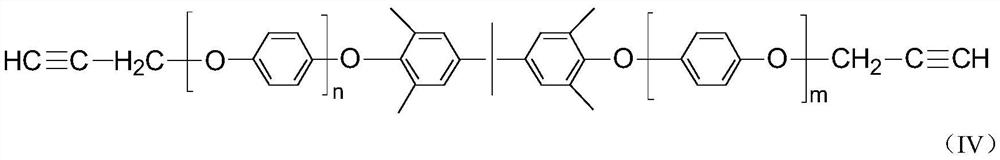
[0030] Put 0.31g of cuprous chloride, 25ml of pyridine, and 50ml of toluene into a 250ml four-neck flask, start mechanical stirring, and maintain the system temperature at 30°C under the protection of oxygen, and it can be observed that the color of the solution turns dark green immediately. In addition, prepare a mixture of 20.18g of 2,6-dimethylphenol, 4.75g of 2,2-bis(4-hydroxy-3,5-dimethylphenyl)propane and 75ml of toluene, and slowly pour it into the above-mentioned four-necked bottle. The mixed solution was added dropwise, and the solution immediately turned yellow-brown. After 2 hours of dropwise addition, the reaction was completed for 4 hours. As the reaction progressed, a pale yellow powder precipitated out. The reaction liquid is poured into 5% hydrochloric acid-methanol solution, and the resin is precipitated, washed with methanol and water, filtered, and dried to obtain a low molecular weight polyphenylene ether. The calculated yield of the low-molecular-weight po...
Embodiment 2
[0033] Put 0.2g of cuprous chloride, 16ml of pyridine, and 40ml of methanol into a 250ml four-neck flask, keep the temperature of the system at 30°C, feed oxygen, start mechanical stirring, and the color of the solution turns dark green immediately. In addition, prepare a mixture of 19.52g of 2,6-dimethylphenol, 4.55g of 2,2-bis(4-hydroxy-3,5-dimethylphenyl)propane and 60ml of methanol, and slowly pour it into the above-mentioned four-necked bottle. The mixed solution was added dropwise, and the solution immediately turned yellow-brown, and the dropwise addition was completed in 2 hours. As the reaction progressed, a pale yellow powder precipitated out, and the reaction was completed in 4 hours. The reaction liquid is directly suction-filtered, and the filter cake is the product, which is then washed with methanol and water, and dried on a heating plate at 120°C to constant weight to obtain low-molecular-weight polyphenylene ether. The calculated yield of the low-molecular-wei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com