Method for producing recombinant vaccine by using plant membrane system
A recombinant vaccine and membrane system technology, applied in the field of recombinant vaccine production using plant membrane systems, can solve the problems of further improvement of the immunogenicity of recombinant vaccines, and achieve easy storage and distribution, good genetic stability, and high immunogenicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Construction of porcine parvovirus surface antigen and chloroplast outer membrane protein fusion vector and Agrobacterium transformation
[0045] 1. Carrier construction
[0046] Synthesize the gene sequence PPV-TOC34 (SEQ ID NO.8) fused with the surface antigen of porcine parvovirus and the transmembrane region of chlorophyll outer membrane protein TOC34, and connect it to the pYBA1132 plant expression vector by double enzyme digestion to obtain the recombinant plasmid DNA (1132-PPV- TOC34). After the ligation product was transformed into Escherichia coli DH5α competent cells, positive clones were obtained by kanamycin screening. Bacterial liquid PCR verified the insertion of PPV-TOC34, and sequenced to further verify the correctness of the sequence. The expression vector map of 1132-PPV-TOC34 is as follows: figure 1 shown.
[0047] 2. Agrobacterium transformation
[0048] Take Agrobacterium GV3101 competent cells (100 μL) stored at -80°C and thaw on ice....
Embodiment 2
[0049] Example 2 Fusion expression of porcine parvovirus surface antigen and chloroplast outer membrane protein
[0050] 1. Preparation of Agrobacterium
[0051] Resuscitate the constructed frozen Agrobacterium, take 5 mL of LB medium with a final concentration of 50 mg / L kanamycin in a 10 mL centrifuge tube, and insert 1% of the inoculated amount, that is, 50 μL of the bacterial solution into the LB medium , 28°C, 200rpm in a shaker for overnight shaking culture. Then expand the culture, take 20 mL of LB medium with a final concentration of 50 mg / L kanamycin in a 50 mL centrifuge tube, insert 1% of the inoculum, that is, 200 μL of the bacterial solution into the LB medium, and set the temperature at 28 ° C and 200 rpm. Cultivate on a shaking table until the OD value of Agrobacterium grows to about 0.5-1.0. Add 20 μL of 100 mmol / L acetosyringone, and continue to shake and incubate for 2 hours.
[0052] 2. Leaf disc transformation
[0053] Select the first fully expanded he...
Embodiment 3
[0054] Example 3 Verification and activity determination of fusion expression of porcine parvovirus surface antigen and chloroplast outer membrane protein
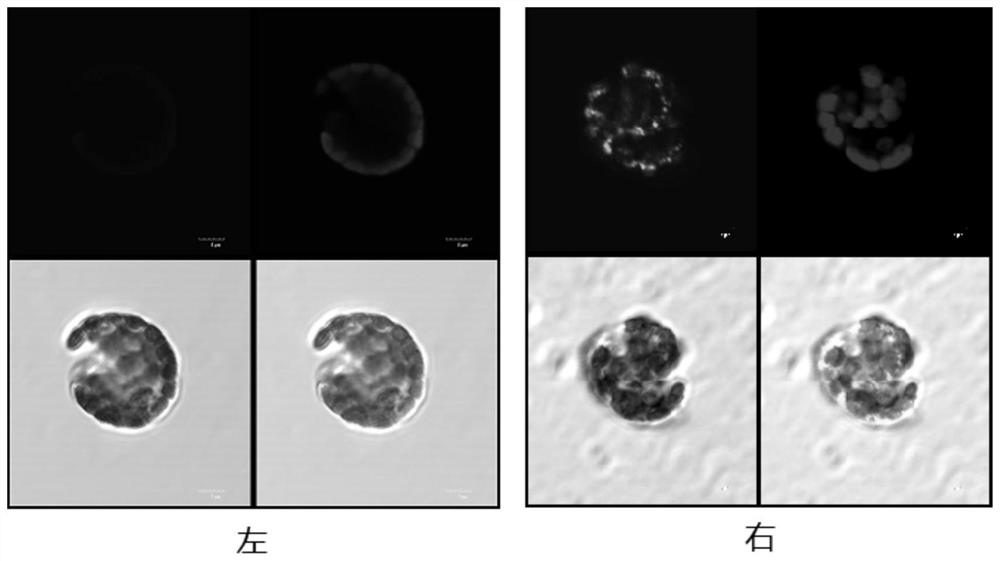
[0055] 1. Immunohistochemistry
[0056] Tobacco leaves are cut into thin shreds, put into the enzymolysis solution (1.5% cellulase, 0.4% pectinase, 0.4M mannitol, 20mM KCl, 20mM MES, 10mM CaCl 2 ) in a petri dish in the dark at 70rpm overnight. The protoplasts were filtered, centrifuged at 60 g, and the supernatant was discarded after centrifugation to retain the precipitate. The pellet was resuspended with 5 mL of WA buffer (Dongsheng Biological) solution. After suspension, use 60g, centrifuge for 10min, discard the supernatant, and keep the precipitate. Add 1 mL of WA buffer solution containing 4% paraformaldehyde to the precipitation, and incubate at room temperature at 60 rpm for 1 h. After immobilization, mouse FITC-labeled anti-PPV monoclonal antibody (VMRD, USA) was incubated. After washing with TBST solution, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com