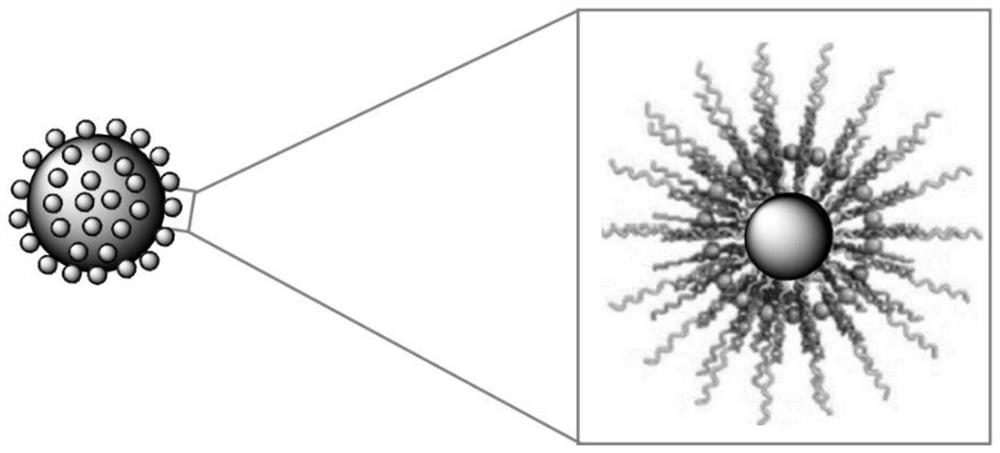
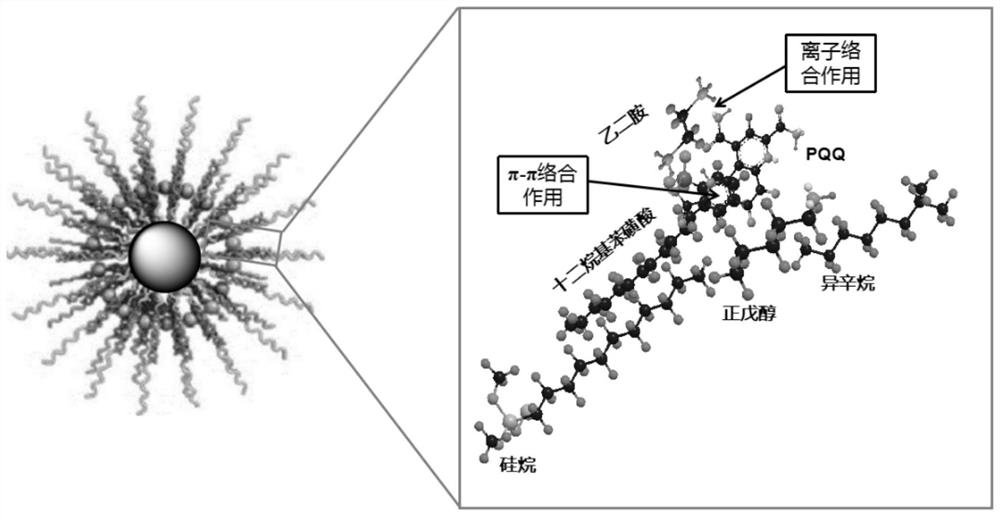
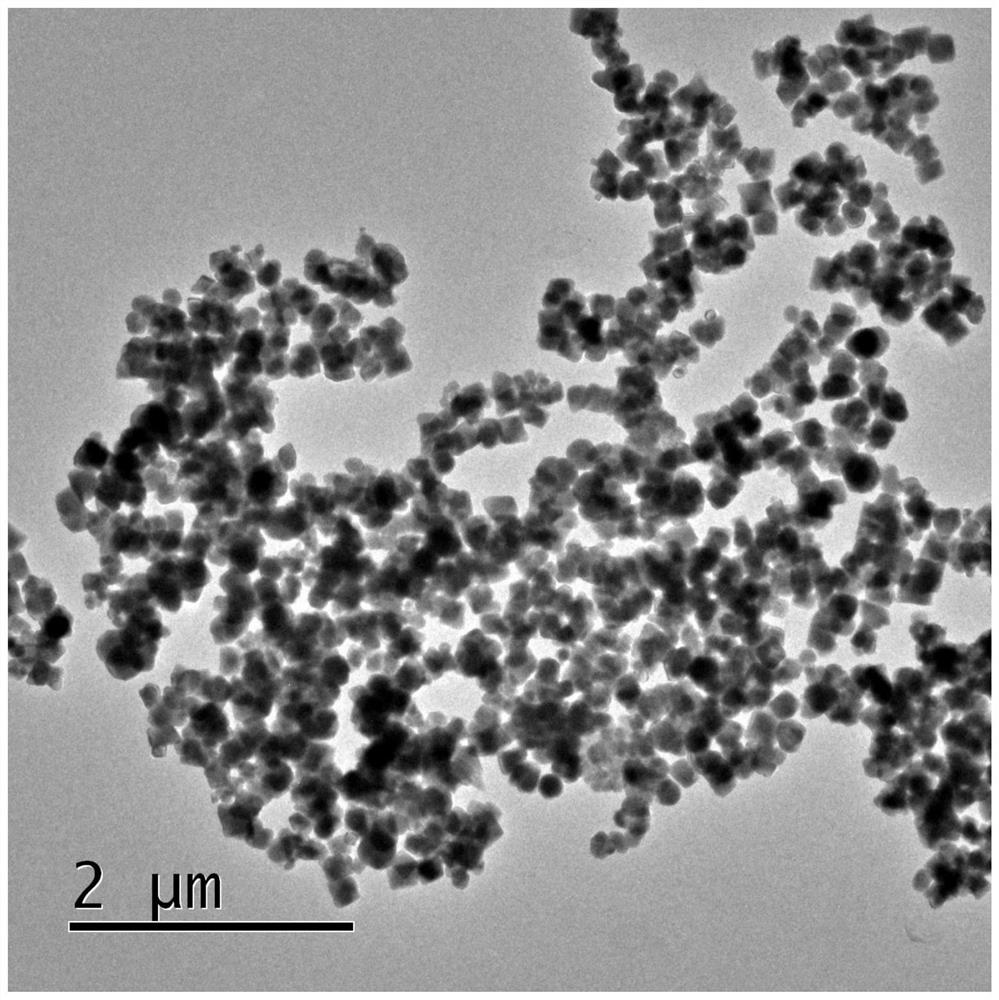
Method for separating and purifying pyrroloquinoline quinone by using reverse micelle surface modified magnetic nano solid-phase extraction material
A solid-phase extraction material and a technology for pyrroloquinoline quinone, which are applied in the field of separation and purification of pyrroloquinoline quinone by magnetic nano solid-phase extraction materials, can solve the problems of low concentration of fermentation products, increased difficulty in separation and purification of PQQ, etc., and achieve mass transfer efficiency. The effect of high, high production efficiency and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of the magnetic nano solid phase extraction material of embodiment 1 reverse micelle surface modification
[0037] A preparation method of magnetic nano solid phase extraction material, comprising the following steps:
[0038] 1) Preparation of Fe by silicon embedding 3 o 4 @SiO 2 ; put 10g Fe 3 o 4 Dissolve in 800mL ethanol solution (consisting of 200mL deionized water and 600mL absolute ethanol), ultrasonically disperse for 30min; then add 10mL 10% (v / v) ammonia water dropwise while stirring, after stirring for 30min, add normal Tetraethyl silicate (TEOS) 60mL, stirred at room temperature for 24h; after the reaction, washed with ethanol for 3 to 5 times to remove the remaining reactants, and then washed with deionized water for 3 to 5 times, and the sample was placed at 60°C Vacuum dry at high temperature for 12h, set aside.
[0039] 2) Fe 3 o 4 @SiO 2 silanization; Take 5mL n-dodecyltrimethoxysilane or n-octadecyltrimethoxysilane and stir at ...
Embodiment 2
[0042] The separation and purification of embodiment 2 pyrroloquinoline quinone
[0043] A method for separating and purifying pyrroloquinoline quinone using a magnetic nanometer solid-phase extraction material modified on the surface of reverse micelles, comprising the following steps:
[0044] (1) Preparation of fermented liquid: Utilize Gluconobacter oxidans to prepare fermented liquid containing pyrroloquinoline quinone, the specific method is:
[0045] Each liter of medium contains 40g sorbitol, 20g yeast extract, 10g (NH 4 ) 2 SO 4 , 4g KH 2 PO 4 , 10gMgSO 4 ·H 2 O.
[0046] Gluconobacter oxydans was inoculated in the fermentation medium according to the inoculum amount of 5%, and cultured with shaking at 28°C for 3 days to obtain the seed liquid; then the seed liquid was inoculated into the enrichment medium according to the inoculum amount of 10%, Shake culture at 28°C for 5 days, centrifuge the culture solution at 5°C and 9000r / min for 15min, collect the super...
Embodiment 3
[0050] The separation and purification of embodiment 3 pyrroloquinoline quinone
[0051] A method for separating and purifying pyrroloquinoline quinone using a magnetic nanometer solid-phase extraction material modified on the surface of reverse micelles, comprising the following steps:
[0052] (1) Preparation of fermented liquid: Utilize Gluconobacter oxidans to prepare fermented liquid containing pyrroloquinoline quinone, the specific method is:
[0053] Each liter of medium contains 40g sorbitol, 20g yeast extract, 10g (NH 4 ) 2 SO 4 , 4g KH 2 PO 4 , 10gMgSO 4 ·H 2 O.
[0054] Gluconobacter oxydans was inoculated in the fermentation medium according to the inoculum amount of 5%, and cultured with shaking at 28°C for 3 days to obtain the seed liquid; then the seed liquid was inoculated into the enrichment medium according to the inoculum amount of 10%, Shake culture at 28°C for 5 days, centrifuge the culture solution at 5°C and 9000r / min for 15min, collect the super...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com