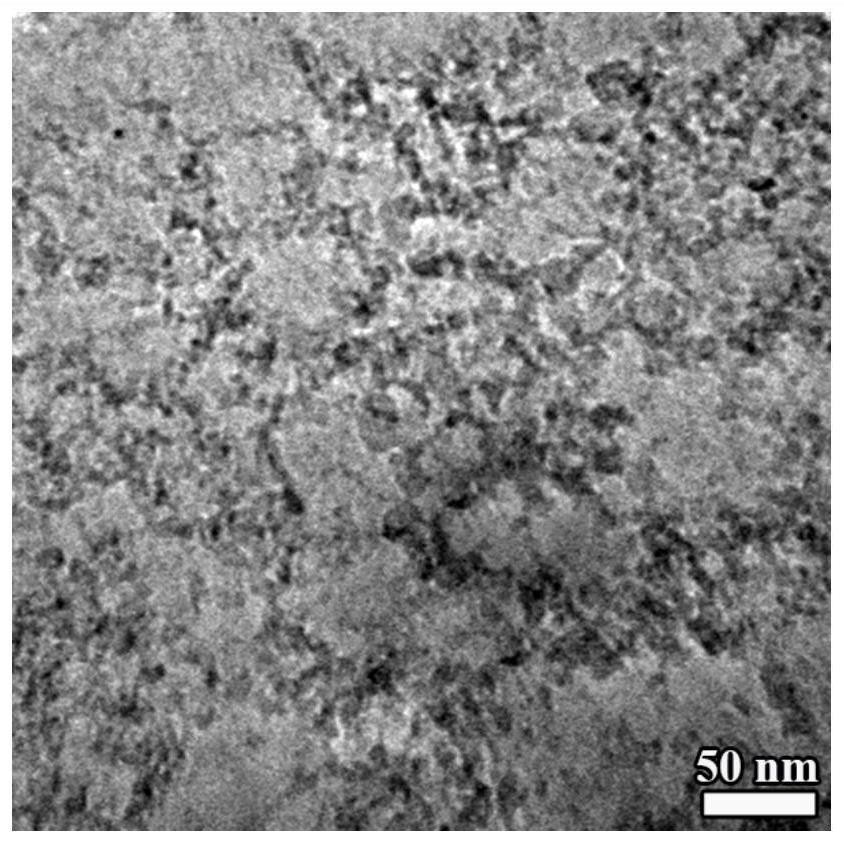
Preparation method of nano-diamond electrolyte and nano-diamond solid electrolyte interface
A nano-diamond and solid electrolyte technology, which is applied in the manufacture of electrolyte batteries, electrode carriers/collectors, non-aqueous electrolyte batteries, etc., can solve problems such as unsuitable for large-scale industrial mass production, harsh conditions in the preparation process, and complex additive structures. To achieve the effect of inhibiting volume expansion, easy to implement, and easy to scale up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of nano-diamond electrolyte
[0037] Take the concentrated hydrochloric acid and the concentrated sulfuric acid solution of 5ml respectively, configure the mixed acid solution that the volume ratio is 1:1; The concentrated hydrochloric acid is the hydrochloric acid solution that commercially available concentration is 36wt%~38wt%; The concentrated sulfuric acid is the concentration 98wt% sulfuric acid solution;
[0038] Add 0.2g (particle size: 5-10nm) of nano-diamond particles into the above mixed acid solution, and heat at 200°C for 30-40min to remove metal impurities on the surface of the diamond particles;
[0039] Take 0.1 g of acid-treated nano-diamond particles, and treat them under ultraviolet (BZS250GF-TC) irradiation for 15 s (15-20 s are acceptable) to obtain oxygen-terminated nano-diamond particles;
[0040] Commercial 1mol / L LiPF 6 (EC:DMC=1:1) The electrolyte is recorded as sample 1, take 0.024g of oxygen-terminated nano-dia...
Embodiment 2
[0042] Embodiment 2: the making of lithium ion battery
[0043] The lithium-ion battery negative electrode is composed of 80wt% commercial graphite (active material), 10wt% binder (polyvinylidene fluoride, PVDF) and 10wt% conductive aid carbon black.
[0044] The three were mixed and ground for 0.5 h, then put into a container, and a certain amount of 1-methyl-2-pyrrolidone (NMP, solvent) was added into the container, and then placed on a magnetic stirrer and stirred at a constant speed for 6 h, until the mixture became a viscous fluid.
[0045] Copper foil is used as a current collector, and the above-mentioned mixed viscous material is coated on the copper foil, and the coating density must be uniform.
[0046] The temperature of the vacuum drying oven was set at 120° C., and the above-mentioned copper foil smear was taken and placed in the drying oven. After timing for 12 hours, it was taken out for use.
[0047] The prepared copper foil smear is cut into several electrode...
Embodiment 3
[0051] Embodiment 3: the test of lithium-ion battery and the formation of nano-diamond solid electrolyte interface
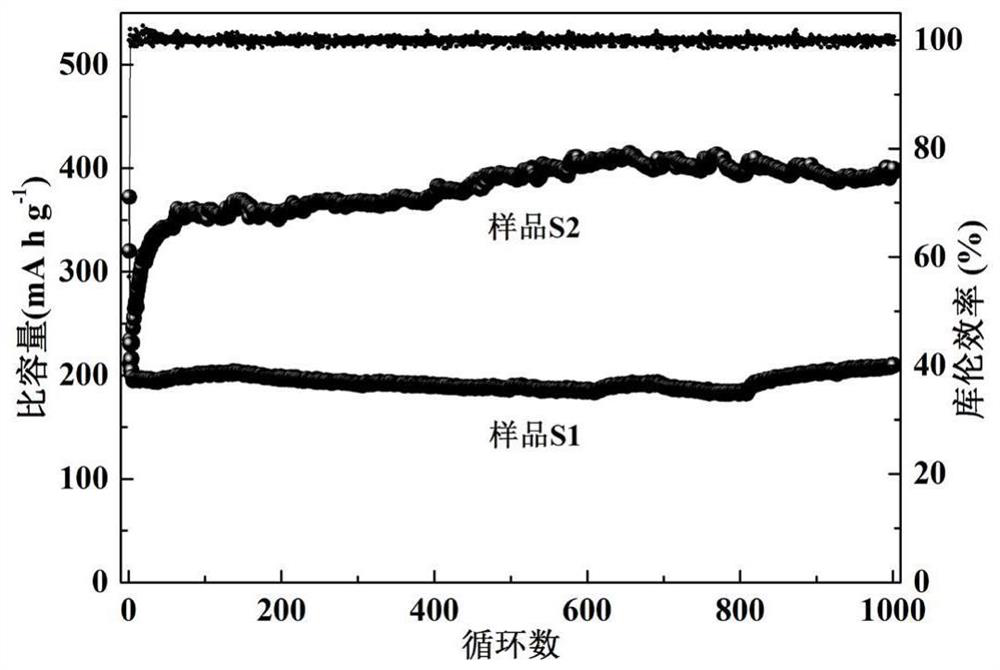
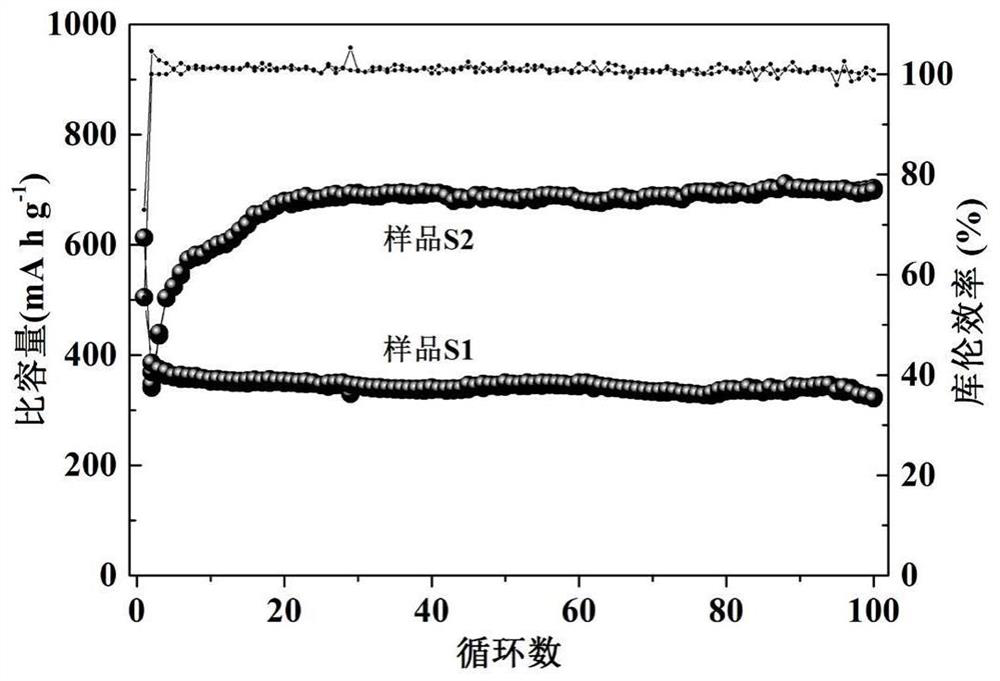
[0052] The electrochemical performance of batteries S1 and S2 was tested in the blue electric test system. At 25°C, discharge to 0.01V at a certain rate; after discharge, let the battery stand for 3 minutes; then charge to 3V at a certain rate, after charging, discharge the battery at the same constant rate for 3 minutes to 0.01V; after discharging the battery, let it stand for 3 minutes, and then charge it under the same conditions. After the test was completed, the active substances in S1 and S2 were taken out and recorded as sample 3 and sample 4, and were characterized by a transmission electron microscope (JEM-2100F, JEOL).
[0053] Testing and Characterization
[0054] 1) Charge and discharge performance test
[0055] The electrochemical performance test results at 5C rate are as follows: figure 2 It can be seen from the figure that the initial discha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com