Preparation method of related impurities of ketorolac or salts thereof
A technology of ketorolac and ketorolac, which is applied in the direction of organic chemistry, can solve the problems of numerous by-products, cumbersome reaction process, and high cost, and achieve the effects of convenient and easy-to-obtain raw materials, simple preparation methods, and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
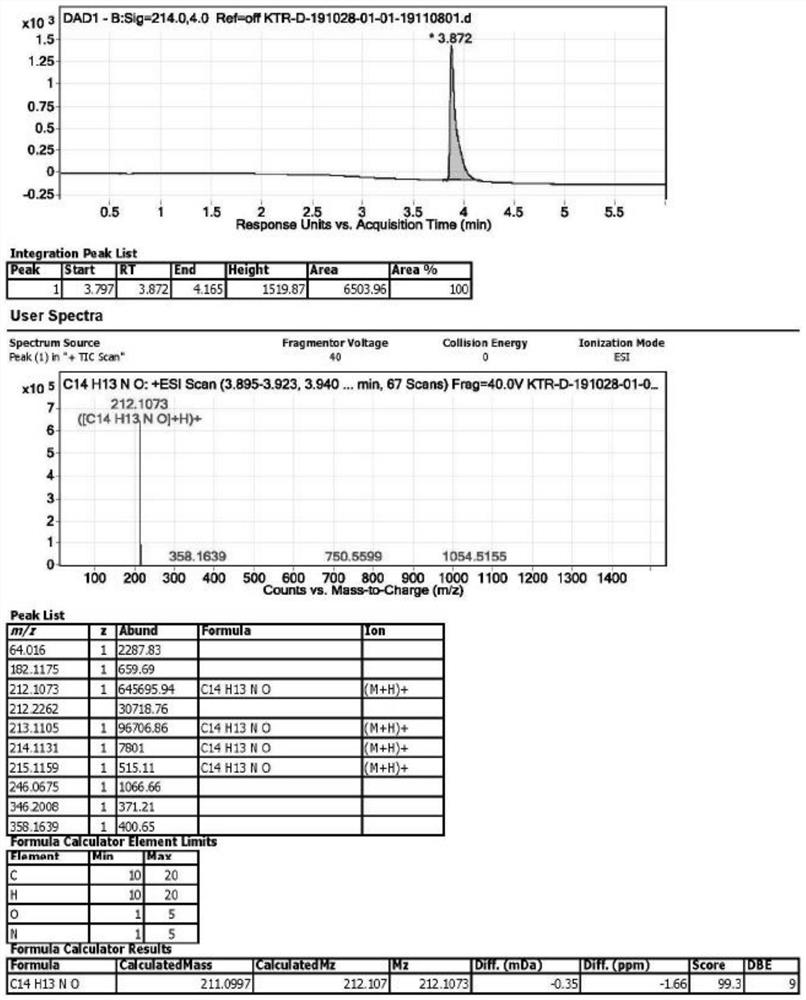
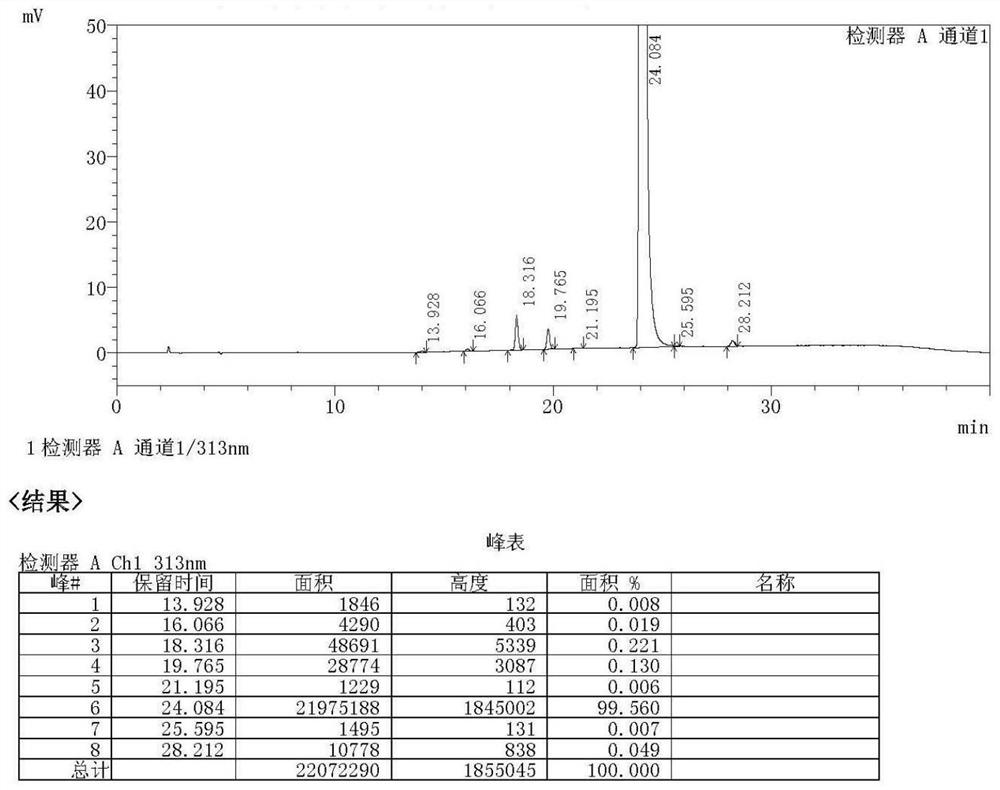
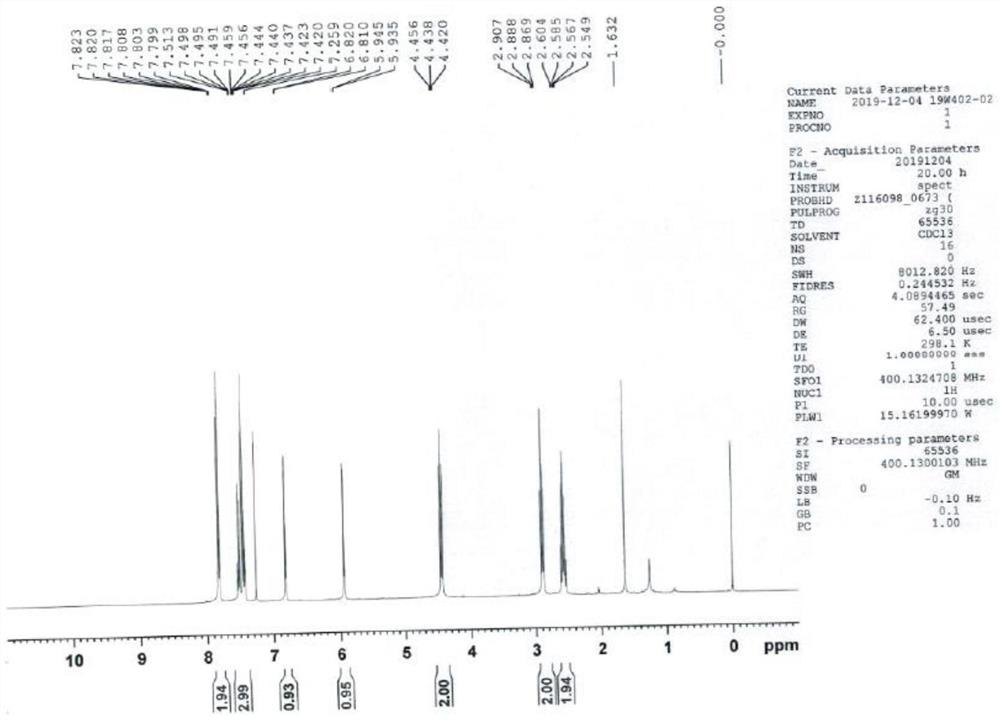
[0042] Take a 500ml single-neck bottle, add 20g of ketorolac, add 100g of DMF (ie dimethylformamide), add 5g of copper powder, and react with magnetic stirring at 140°C for 12h, TLC (ie, thin layer chromatography) to monitor the reaction progress. 40°C, filter to remove excess copper powder, add 200 g of water, the system becomes turbid, extract three times with 50 g of ethyl acetate each time, combine the organic phases and concentrate to dryness to obtain a pale yellow crystalline solid 6,7-dihydro-5H-pyrrolizine-3 -Base (phenyl) ketone 15.2g (the specific structure confirms the map to participate in Figure 1-3 ), purity 99.56%, yield 92%.
[0043] Take a 500ml single-neck bottle, add 5g of 6,7-dihydro-5H-pyrrolizin-3-yl (phenyl) ketone, add 50g of acetic acid, add 20g of 20% potassium permanganate aqueous solution, control the temperature to 80°C, and magnetically stir 6h, TLC monitored the reaction progress and the reaction was completed, cooled to 25°C, poured into 200g...
Embodiment 2
[0046] Take a 500ml single-neck bottle, add 20g of ketorolac, add 100g of ethylene glycol, add 6g of iron powder, and react with magnetic stirring at 150°C for 12h. TLC monitors the reaction progress and the reaction is completed. After the reaction is completed, the temperature is lowered to 40°C, the excess iron powder is removed by filtration, and 200g of water is added. The system became cloudy, extracted three times with 50 g of ethyl acetate each time, and the combined organic phases were concentrated to dryness to obtain 12.06 g of pale yellow crystalline solid 6,7-dihydro-5H-pyrrolizin-3-yl(phenyl)methanone, with a purity of 12.06 g. 99.43%, yield 73%.
[0047] Take a 500ml single-neck bottle, add 5g of 6,7-dihydro-5H-pyrrolizin-3-yl (phenyl) ketone, add 50g acetic acid, add 20g 20% high chromium trioxide aqueous solution, control the temperature to 70°C, and stir magnetically for 3h , TLC monitored the reaction progress and the reaction was completed, cooled to 25°C...
Embodiment 3
[0050] Take a 500ml single-neck bottle, add 20g of ketorolac, add 100g of p-xylene, add 5g of copper powder, magnetic stirring and reflux for 16h, TLC to monitor the reaction progress and complete the reaction, cool down to 40°C, filter to remove excess copper powder, add 200g of water and the system becomes cloudy , extracted three times with 50 g of ethyl acetate each time, and the combined organic phases were concentrated to dryness to obtain a pale yellow crystalline solid 6,7-dihydro-5H-pyrrolizin-3-yl (phenyl) ketone 6.58 g with a purity of 99.24%, Yield 59%.
[0051] Take a 500ml single-neck bottle, add 5g of 6,7-dihydro-5H-pyrrolizin-3-yl (phenyl) ketone, add 50g of acetic acid, add 20g of 20% ammonium cerium nitrate aqueous solution, control the temperature to 80°C, and stir magnetically for 3h , TLC monitored the reaction progress and the reaction was completed, cooled to 25°C, poured into 200g ice water to quench the reaction, the system changed from reddish-brown t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com