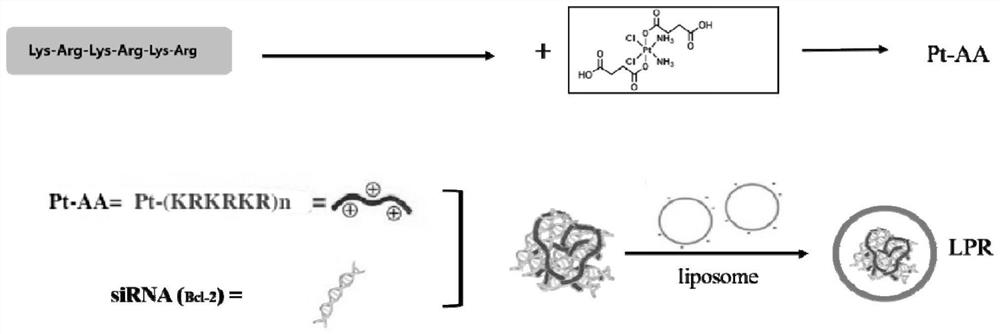
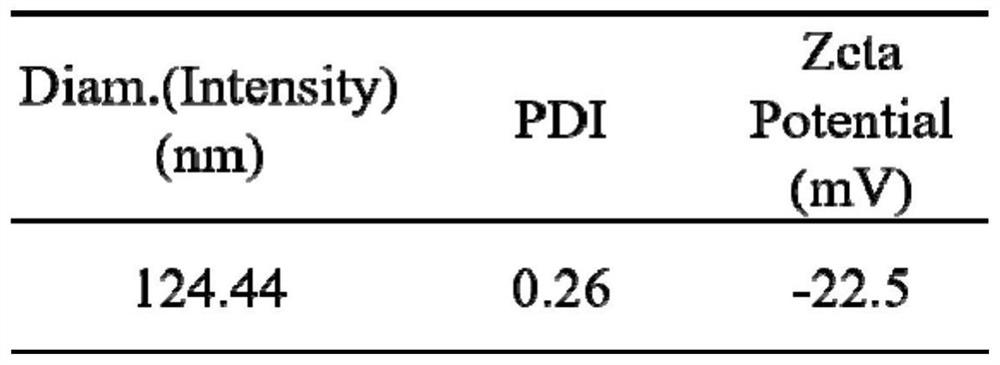
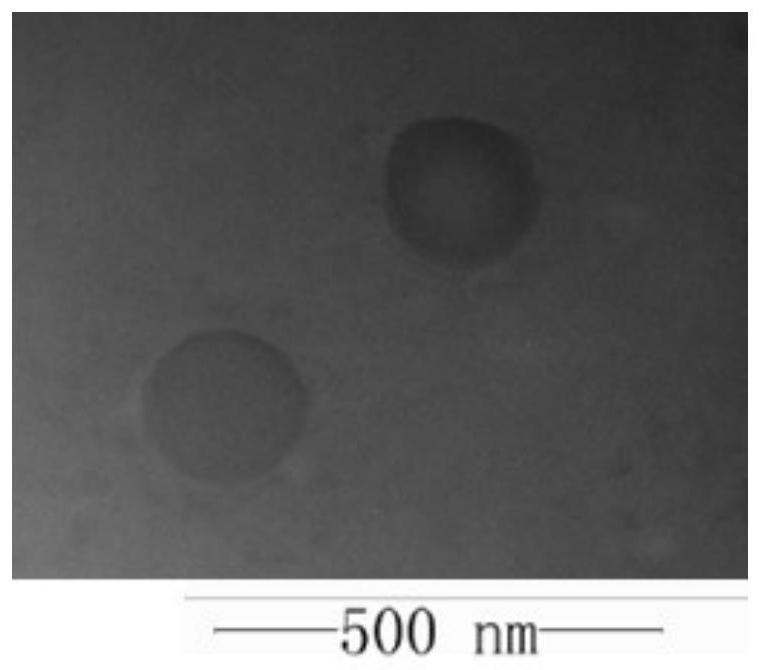
Preparation method and application of platinum peptide copolymer with siRNA transportation function
A copolymer and functional technology, applied in the field of biomedical applications, can solve problems such as leakage, affecting the size of the carrier structure, load capacity, side effects, etc., to achieve the effects of reducing losses, avoiding non-specific leakage, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Solid phase synthesis of polypeptide KRKRKR (Lys-Arg-Lys-Arg-Lys-Arg)
[0050] 1a. Weigh 500mg Rink Amide MBHA resin, add DMF (N,N-dimethylformamide) to swell for 1h; add 5ml 20% piperidine / DMF solution to react for 10min, then wash with DMF solution 4 times; add 700mgFomc-Lys (Boc)-OH, 180mg 1-hydroxybenzotriazole (HOBT), 520mg benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU) and 450ul N,N -Diisopropylethylamine (DIEA), dissolved in DMF for 12 hours, washed with DMF; then carried out to detect the amino group with ninhydrin, showing that it did not turn blue, indicating that the connection was successful; adding 8ml of 20% piperidine / DMF solution to react for 10min, and This step (adding 8ml of 20% piperidine / DMF solution to react for 10min) was repeated once, washed with DMF, and then carried out ninhydrin to detect the amino group, and the display turned blue, indicating that the deprotection was successful.
[0051] 1b. Add 750mg ...
Embodiment 2
[0054] Embodiment 2: the synthesis of tetravalent carboxyplatinum
[0055] 2a. Weigh 100mg of cisplatin into a 50ml round bottom flask, then add 7-8ml of 30% H 2 o 2 Solution, stirred and reacted for 4 hours at 75°C in the dark; to complete the reaction, remove most of the solvent by rotary evaporation, then cool overnight, collect the precipitate, wash with acetone and ether for 1-2 times, and freeze-dry to obtain hydroxyplatinum;
[0056] 2b. Weigh 100mg of hydroxyplatinum and 120mg of succinic anhydride into a 50ml round bottom flask, add 3-5ml of DMF, and stir at 50°C in the dark for 24h; end the reaction, remove the solvent by lyophilization, dissolve with a little acetone, add ether dropwise to precipitate out , the precipitate was collected, washed with ether three times, and freeze-dried to obtain the tetravalent carboxyplatinum product Pt(IV)-COOH.
Embodiment 3
[0057] Embodiment 3: the synthesis of platinum peptide cross-linked product Pt-AA
[0058] Weigh 74 mg of the polypeptide KRKRKR and dissolve it in 15 ml of 100 mM PBS with pH=7.4, then add 2 times the molar amount (calculated as the polypeptide) of Pt(IV)-COOH, 8 times the molar amount (calculated as the polypeptide) of NHS and 20 times the molar amount ( Calculated by polypeptide) EDCI was dissolved in 5ml water and reacted for 30min, then the two solutions were mixed evenly, stirred at room temperature in the dark for 12h, after lyophilization, the solvent was removed, firstly purified with chloroform and acetone to remove organic small molecule impurities, and then passed through gel chromatography The column removes impurities to obtain the platinum peptide cross-linked product Pt-AA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com