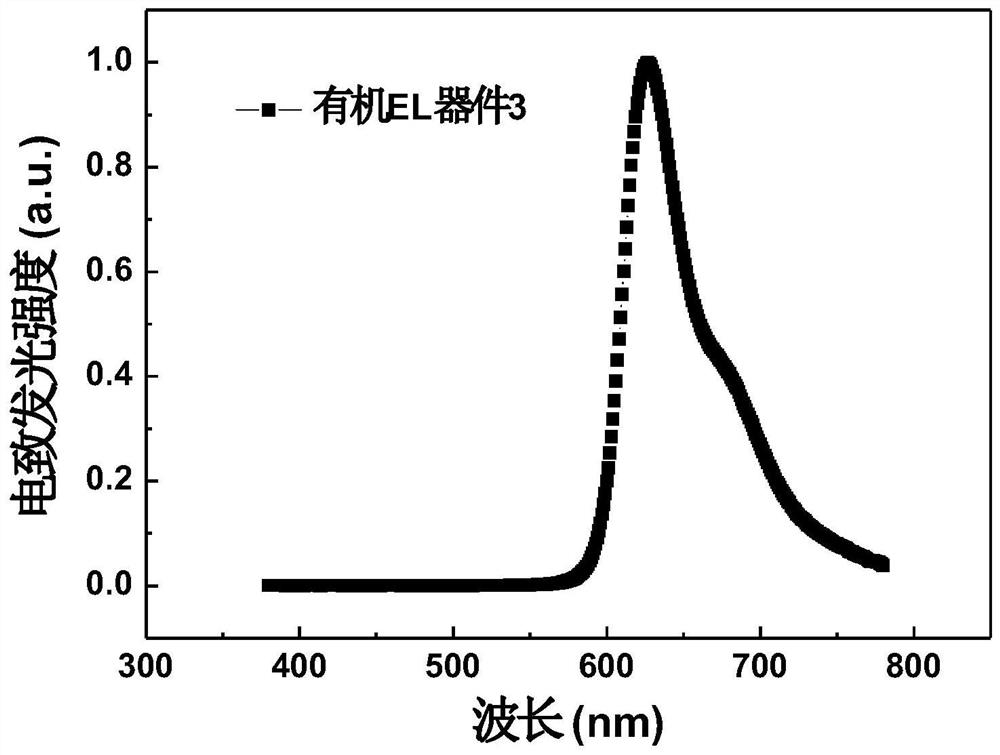
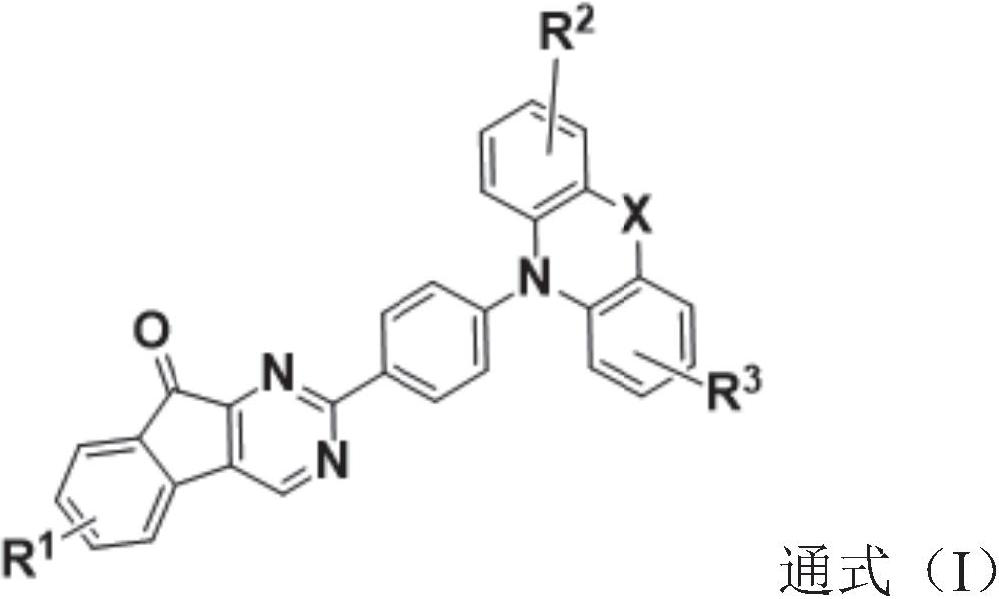
1, 3-diazafluorenone derivative and electronic device
A technology of electronic devices and heterofluorenones, which is applied in the field of organic photoelectric materials, can solve the problems of device electron and hole mobility imbalance, device efficiency and life reduction, molecular structure or crystal state changes, etc., and achieve excellent hole blocking The ability to reduce the driving voltage and the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of compound 1-7
[0043] (Synthesis of intermediate M1)
[0044] The synthetic route of intermediate M1 is as follows:
[0045]
[0046]Add 2-(4-bromophenyl)-1,3-diazafluorene (6.46g, 20mmol), chromium trioxide (2.5g, 25mmol) and 120mL acetic acid successively in a 250mL single-necked flask, and react under reflux for 4 hours . After the reaction was completed, it was cooled to room temperature. The reaction solution was poured into water and extracted with dichloromethane. The organic phase was washed with saturated sodium bicarbonate solution. The organic phase was dried and evaporated under reduced pressure, and the obtained crude product was further purified by column chromatography (petroleum ether:dichloromethane=2:1 (V / V)). The solvent was evaporated, and after drying, 3.50 g of a yellow solid was obtained with a yield of 52%. MS(EI): m / z: 337.02[M + ]. Anal.calcd for C 17 h 9 BrN 2 O (%): C60.56, H2.69, N 8.31; found: C ...
Embodiment 2
[0051] Embodiment 2: the synthesis of compound 2-2
[0052] The synthetic route of compound 2-2 is as follows:
[0053]
[0054] Under nitrogen protection, intermediate M1 (1.69g, 5mmol), bis(4-biphenyl)amine (1.64g, 5.1mmol), palladium acetate (22.4mg, 0.1mmol), three tert-butylphosphinetetrafluoroborate (73mg, 0.25mmol), sodium tert-butoxide (1.0g, 10mmol) and 120mL toluene were stirred under reflux for 12 hours. After the reaction was complete, the solvent was evaporated, the residue was dissolved with 200 mL of dichloromethane, washed with water, the organic layer was separated, the aqueous layer was extracted twice with 15 mL of dichloromethane, and the organic layers were combined. After distilling off the solvent, the residue was separated by column chromatography (petroleum ether:dichloromethane=2:1 (V / V)). The solvent was evaporated, and after drying, 2.25 g of an orange-red solid was obtained, with a yield of 78%. MS (EI): m / z: 577.50 [M + ]. Anal.calcd for C...
Embodiment 3
[0055] Embodiment 3: the synthesis of compound 3-1
[0056] The synthetic route of compound 3-1 is as follows:
[0057]
[0058] Under nitrogen protection, intermediate M1 (1.69g, 5mmol), 9,10-dihydro-9,9-dimethylacridine (1.05g, 5.1mmol), palladium acetate (22.4 mg, 0.1 mmol), tri-tert-butylphosphine tetrafluoroborate (73 mg, 0.25 mmol), sodium tert-butoxide (1.0 g, 10 mmol) and 120 mL of toluene, refluxed and stirred for 12 hours. After the reaction was complete, the solvent was evaporated, the residue was dissolved with 200 mL of dichloromethane, washed with water, the organic layer was separated, the aqueous layer was extracted twice with 15 mL of dichloromethane, and the organic layers were combined. After distilling off the solvent, the residue was separated by column chromatography (petroleum ether:dichloromethane=2:1 (V / V)). The solvent was evaporated, and after drying, 1.63 g of an orange-yellow solid was obtained, with a yield of 71%. MS (EI): m / z: 465.58 [M +...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com