Pyrene-based aromatic amine compound as well as preparation method and application thereof as organic electroluminescent material
A compound and aromatic amine technology, applied in the field of organic electroluminescent materials, can solve problems such as wide emission spectrum, poor color purity, and poor luminous efficiency, and achieve the effects of improving luminous efficiency, reducing driving voltage, and prolonging device life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention provides a preparation method of the above-mentioned pyrene-based aromatic amine compound, comprising the following steps:
[0042] (1) Under the action of a phosphine-palladium catalyst, a compound having a structure shown in formula a and a compound having a structure shown in formula b carry out the first Suzuki reaction to obtain a compound having a structure shown in formula c;
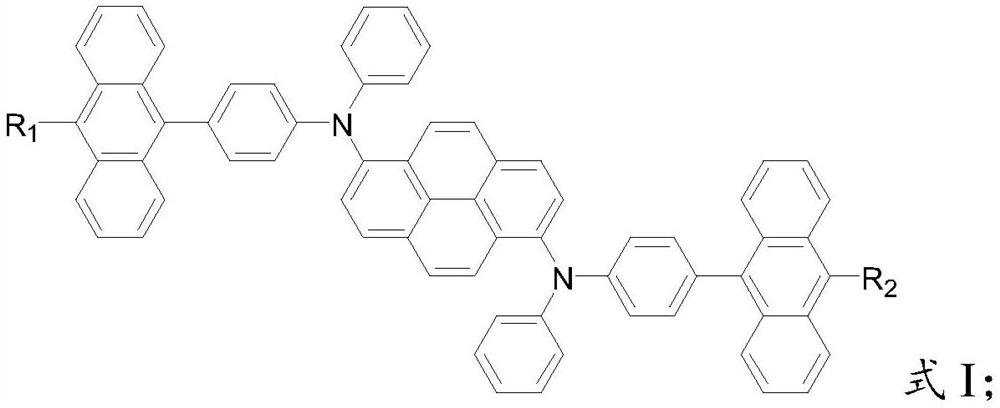
[0043] Formula a; in formula a, R is an unsubstituted or alkyl-substituted phenyl group, and the alkyl group in the alkyl-substituted phenyl group is a C1-C9 alkyl group;
[0044]
[0045] (2) a compound having a structure shown in formula c is subjected to a halogenation reaction with bromosuccinimide to obtain a compound having a structure shown in formula d;
[0046]
[0047] (3) A compound having a structure shown in formula d is mixed with n-butyllithium and trimethyl borate, and subjected to a lithiation reaction to obtain a compound having a structure s...
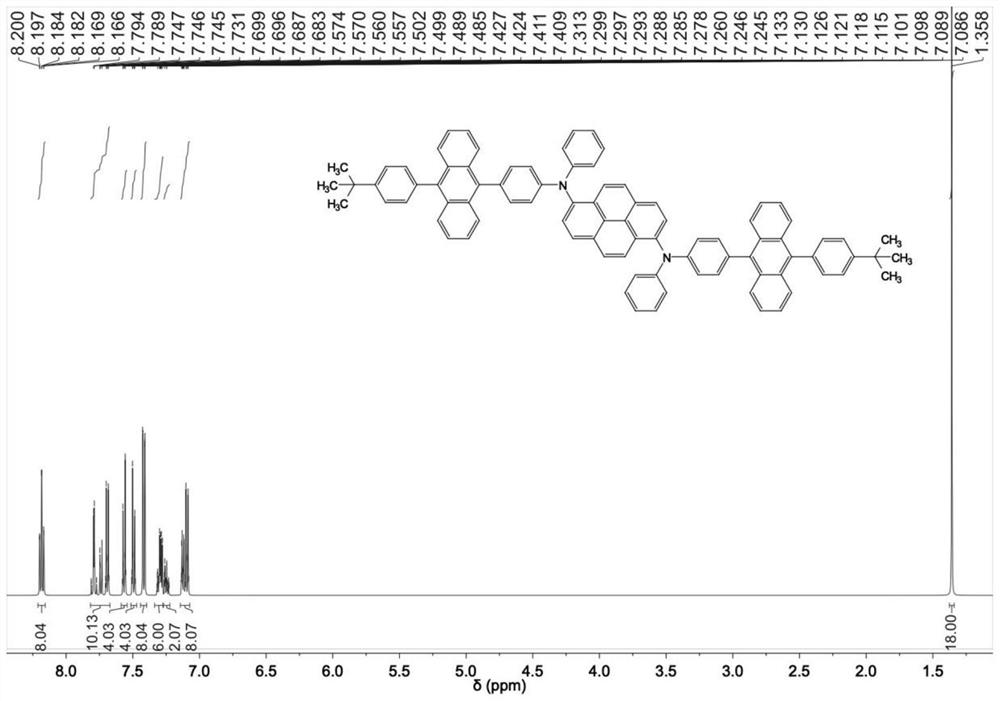
Embodiment 1
[0106] Preparation of pyrene-based aromatic amine compound I-6
[0107] The first step: the preparation of intermediate 1
[0108]
[0109] In the reaction bottle, add 100mmol raw material 1, 100mmol raw material 2, 200mmol potassium carbonate, 200mL toluene, 100mL ethanol and 10mL deionized water, add 0.5g tetrakistriphenylphosphine palladium under nitrogen atmosphere, react at 100 degrees for 10 hours, cool , filtered, and the product was recrystallized from toluene with a yield of 91%.
[0110] The second step: the preparation of intermediate 2
[0111]
[0112]In the reaction bottle, add 70mmol intermediate 1, 73mmol bromosuccinimide, 180ml DMF, react at room temperature for 12 hours, add 550mL deionized water, filter, dry, and recrystallize with toluene, the yield is 86%.
[0113] The third step: the preparation of intermediate 3
[0114]
[0115] In the reaction bottle, add 50mmol of intermediate 2, 150ml of anhydrous tetrahydrofuran, under the protection of ...
Embodiment 2
[0127] Preparation of organic electroluminescent devices OLEDs using compound 6 as blue light material: device preparation equipment: MB-MO-SE1 vacuum thermal evaporation coating equipment from Mbraun, Germany; testing equipment: Keithley Source 2400, PhotoResearchPR655 spectrometer.
[0128] The structure of the device is: ITO / HAT-CN(10nm) / NPB(50nm) / BH:8% Compound I-6(30nm) / TmPyPB(40nm) / LiF(1nm) / Al(100nm)
[0129] The compounds used in the device are all commercial products, and the structural formula is as follows:
[0130]
[0131] The specific preparation steps of the device are as follows:
[0132] Use 10Ω / sq ITO glass as the substrate, clean it with glass cleaning agent first, then use deionized water and acetone to sonicate 3 times each, and after UV-ozone treatment for 15 minutes, the organic layer is evaporated. First, 10nm hole injection material HAT-CN was deposited on the ITO glass substrate, then 50nm NPB was deposited as a hole transport layer, and then the l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com