Procaine penicillin, preparation method thereof, impurity and impurity control method
A technology of procaine penicillin and a control method, which is applied in the field of drug preparation and can solve the problems of difficulty in quality monitoring, unclear impurities of procaine penicillin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
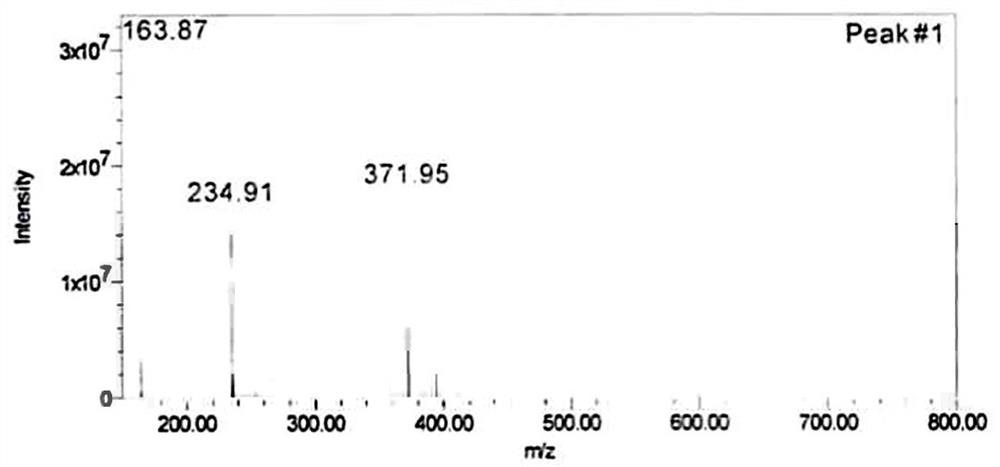
[0032] A procaine penicillin impurity is characterized as a compound shown in formula (1), and the compound English name: 2-{[2-(4-aminobenzoyloxy)ethyl](ethly)amino}ethyl-4-aminobenzoate. Chinese name: 2-{[2-(4-aminobenzoyloxy)ethyl](ethyl)amino}ethyl-4-aminobenzoate. Molecular formula: C 20 h 25 N 3 o 4 . Molecular weight: 371.
[0033]
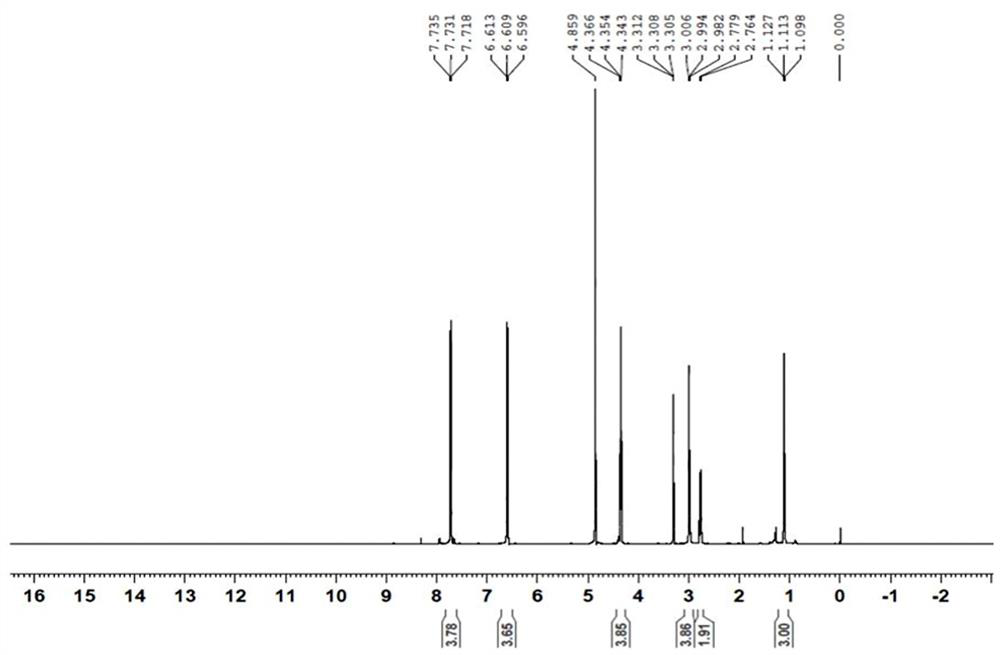
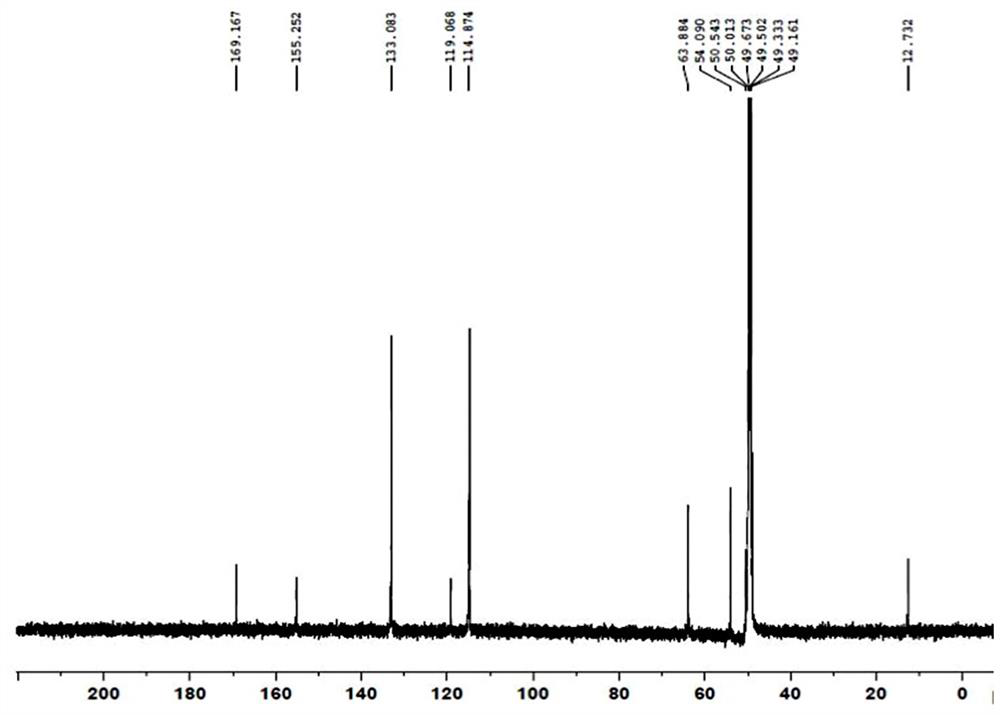
[0034] Structure confirmation: LC-MS analysis of RRT0.24 impurity, [M+H] + =372, the molecular weight is 371, see attached figure 1 . The RRT0.24 impurity was analyzed by nuclear magnetic resonance, the data is shown in Table 1, and the spectrum is attached figure 2 , 3 . Analysis of hydrogen spectrum data: (1) There is one methyl group in the structure: it is adjacent to the methylene group (triple peak), and the chemical shift is δ1.11ppm. (2) There are 5 methylene groups in the structure: adjacent to heteroatoms, they are divided into 3 groups of signals, and the chemical shifts are between δ2.7-4.4ppm. (3) There are 8 a...
Embodiment 2
[0040] The synthesis process of the procaine penicillin RRT0.24 impurity is as follows:
[0041]
[0042]The impurity is introduced by the raw material procaine hydrochloride and is generated during the synthesis of procaine hydrochloride. In the synthesis process of procaine hydrochloride, diethylamine is used to add ethylene oxide to obtain diethylaminoethanol, and then form an intermediate with p-nitrobenzoic acid through esterification, and then reduce to obtain procaine. Due to the presence of ethylamine in the starting material diethylamine, N,N'-di(2-ethanolyl)ethylamine is generated during the addition of ethylene oxide, and further undergoes esterification reaction with two molecules of p-nitrobenzoic acid , post-reduction produces RRT0.24 impurity. The production process of procaine penicillin does not have the conditions for the formation of this impurity.
Embodiment 3
[0044] Preparation of procaine penicillin:
[0045] (1) prepare procaine hydrochloride solution: detect the content of RRT0.24 impurity in raw material procaine hydrochloride, its content is 0.036%, 15.3g procaine hydrochloride is added in 41mL purified water under room temperature, ultrasonically dissolves to After clarification, add water to 53mL.
[0046] (2) Preparation of penicillin potassium salt buffer: 0.47g buffer salt (Na 2 HPO 4 and NaH 2 PO 4 The mass ratio is 6:1 or 0.40g Na 2 HPO 4 +0.067g NaH 2 PO 4 ) into 61mL of purified water, then 0.067g of NaCl, ultrasonically dissolved until clarified, at room temperature, add 20g of penicillin potassium salt, ultrasonically dissolved until clarified, and replenish water to 93mL.
[0047] (3) Crystallization unit: slowly add 0.2mL procaine hydrochloride solution and 0.024g seed crystal into the penicillin potassium salt buffer respectively, and grow the crystal for 7min. Add procaine hydrochloride solution to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com