Indole alkaloid compound as well as preparation method, pharmaceutical composition and application thereof
A technology of indole alkaloids and compositions, which is applied in the direction of drug combinations, organic chemistry, antineoplastic drugs, etc., can solve the problems of no relevant activity and no indole alkaloids, and achieve simple operation, low cost, and low cost of raw materials Effects from a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
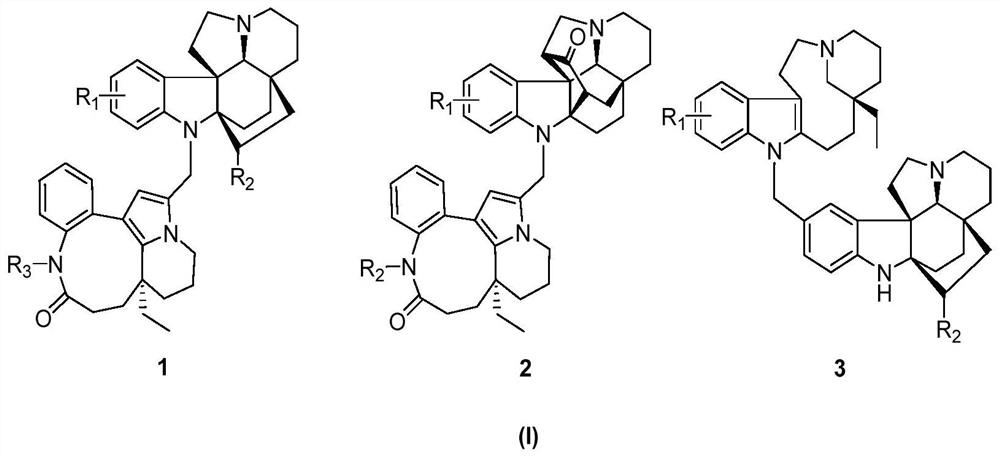
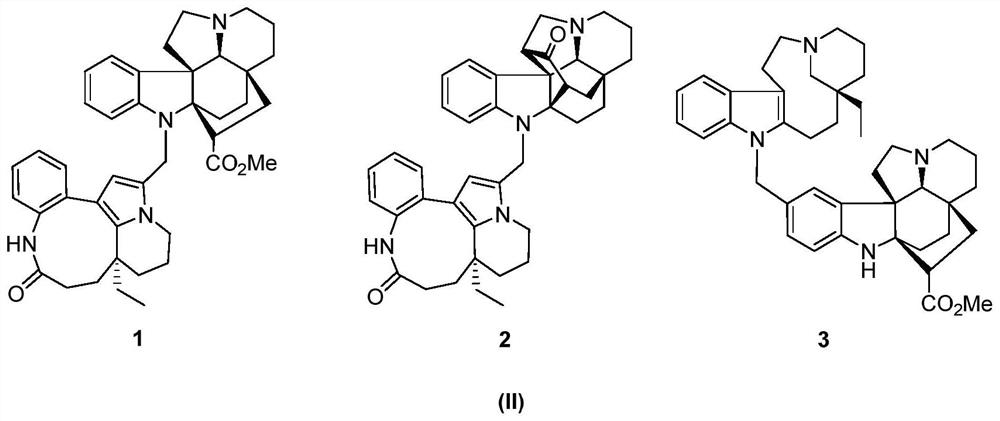
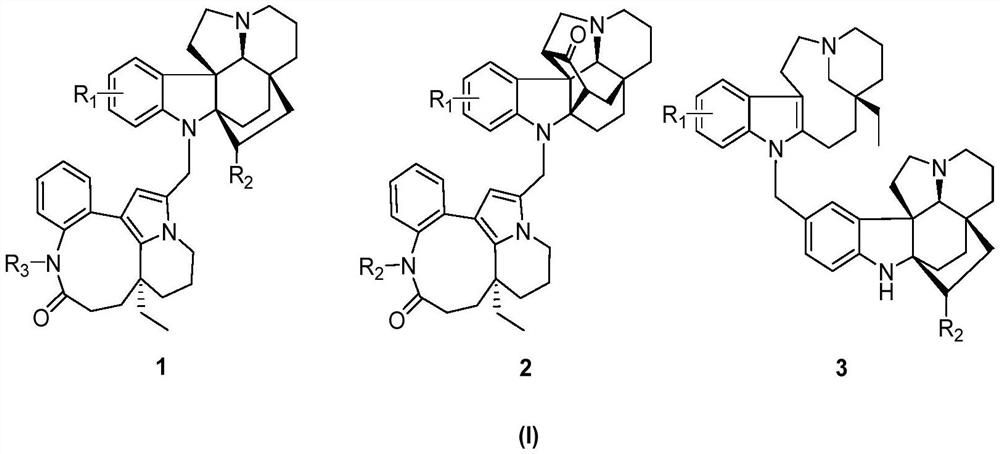
[0054] 1.2 kg of dried seeds of Kopsia arborea, heated and refluxed with methanol to extract three times, each time for 3 hours, combined the extracts, recovered methanol under reduced pressure, kneaded the concentrated extract with 10% hydrochloric acid aqueous solution and adjusted the pH value 2 to 3, extract three times with petroleum ether, then adjust the pH value to 9 to 10 with 10% NaOH solution, and use CH 2 Cl 2 Extraction obtains total base part 154g, total base part utilizes silica gel column chromatography, petroleum ether-acetone gradient elution obtains five parts (Fr.A-E), Fr C (38g) utilizes silica gel column chromatography, petroleum ether / acetone (10: 1→5:1, v / v), reverse phase chromatography C-18 (acetonitrile / water, 30:70→80:20) and SephadexLH-20 (methanol) separate and obtain the indole alkaloid 1 of structure shown in formula II (6.5mg, yield 0.004%, purity 98%, colorless crystals), 2 (12mg, yield 0.008%, purity 98%, pale yellow oil) and 3 (8.7mg, yield...
Embodiment 2
[0071] Mix the indole alkaloid prepared in Example 1 and water for injection evenly, perform fine filtration, potting and sterilization in sequence to obtain the indole alkaloid injection.
Embodiment 3
[0073] Preparation of medically acceptable salts of indole alkaloids:
[0074] The indole alkaloids prepared in Example 1 are respectively reacted with tartaric acid, citric acid, formic acid, acetic acid, oxalic acid, hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid with a mass concentration of 4%, to obtain the tartaric acid of indole alkaloids respectively Salt, citrate, formate of indole alkaloid, acetate of indole alkaloid, oxalate of indole alkaloid, hydrochloride of indole alkaloid, hydrogen bromide of indole alkaloid Salts of indole alkaloids, nitrates of indole alkaloids, sulfates of indole alkaloids.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com