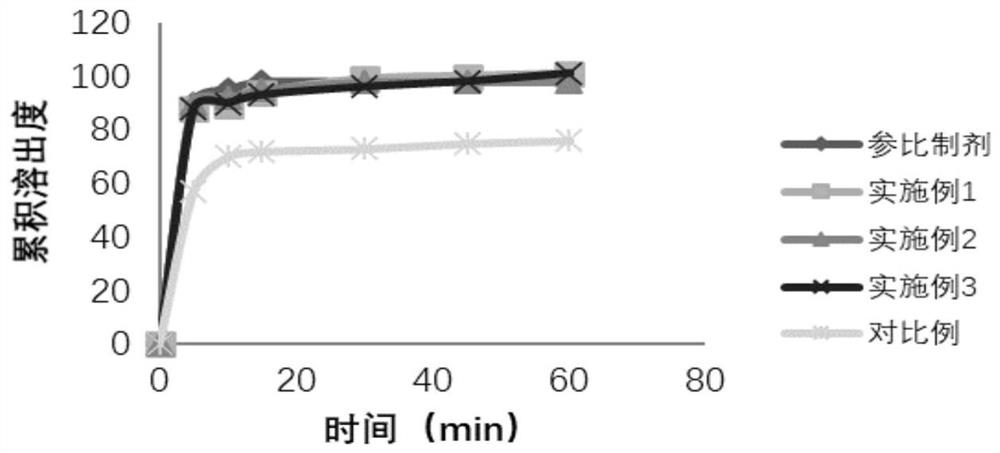
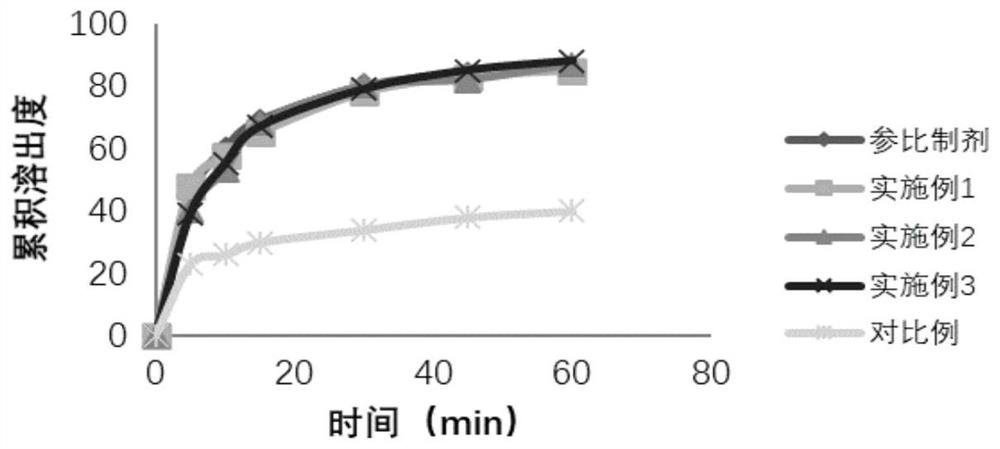
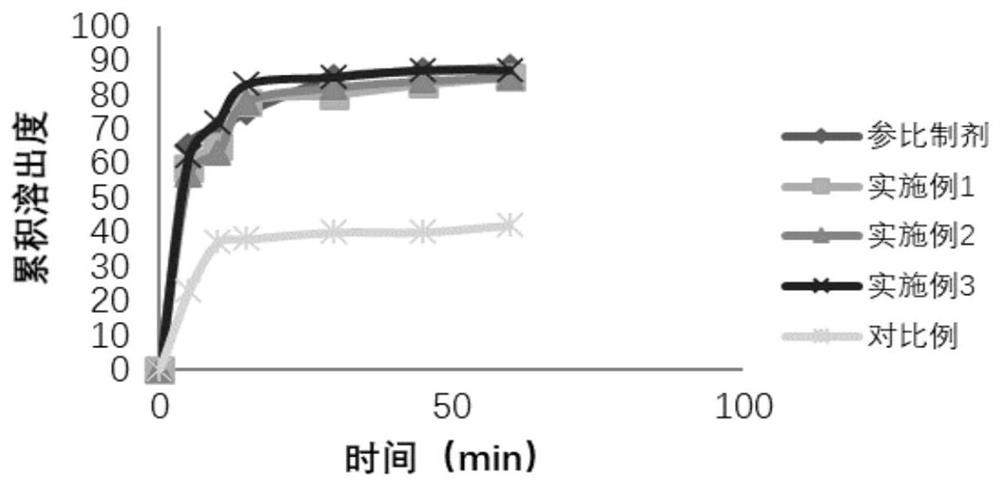
Preparation method of cefditoren pivoxil granules
A technology for cefditoren pivoxil and granules, which is applied in the field of preparation of cefditoren pivoxil granules, can solve the problems of poor stability of amorphous raw materials, unstable light conditions, and cannot be stored for a long time, and achieves a reduction in the process Difficulty and risk, the effect of saving consumption and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: The composition of the prescription is as follows, the information of the mixed organic solvent: acetone: ethanol: 1:2.
[0027]
[0028] Preparation Process:
[0029] 1) Material pretreatment: sucrose is pulverized through a 80-mesh sieve, and aspartame and crospovidone are all passed through an 80-mesh sieve to remove agglomerates;
[0030] 2) Adhesive configuration: Mix acetone and ethanol evenly in a ratio of 1:2, then add the prescribed amount of raw materials, hypromellose, and sodium chloride, and stir until completely dissolved;
[0031] 3) Granulation: Add the prescribed amount of sucrose, crospovidone, and aspartame into the fluidized bed, use the prepared binder for top-spray granulation, control the inlet air temperature at 50-80°C, and The wind frequency is 30-50HZ, the atomization pressure is 0.2-0.4Mpa, and the peristaltic pump speed is 20±3rpm;
[0032] 4) Drying: Control the air inlet temperature at 60-80°C, the air inlet frequency at 30...
Embodiment 2
[0035] Example 2: The composition of the prescription is as follows, the information of the mixed organic solvent: the ratio of acetone:ethanol is 1:1.
[0036]
[0037] Preparation Process:
[0038] 1) Material pretreatment: sucrose is pulverized and passed through an 80-mesh sieve, and both croscarmellose sodium and aspartame are passed through an 80-mesh sieve to remove agglomerates;
[0039] 2) Adhesive configuration: Mix acetone and ethanol evenly in a ratio of 1:1, then add the prescribed amount of raw materials, hydroxypropyl cellulose, and sodium chloride, and stir until completely dissolved;
[0040] 3) Granulation: Add the prescribed amount of sucrose, croscarmellose sodium, and aspartame into the fluidized bed, use the prepared binder for top spray granulation, and control the inlet air temperature at 60-90 ℃, air intake frequency 35-50HZ, atomization pressure 0.3-0.4Mpa, peristaltic pump speed 20±3rpm;
[0041] 4) Drying: Control the air inlet temperature at 6...
Embodiment 3
[0044] Example 3: The composition of the prescription is as follows, the information of the mixed organic solvent: the ratio of acetone:ethanol is 2:1.
[0045]
[0046] Preparation Process:
[0047] 1) Material pretreatment: sucrose is crushed and passed through an 80-mesh sieve, carboxymethyl starch sodium and sucralose are all passed through an 80-mesh sieve to remove agglomerates;
[0048] 2) Adhesive configuration: Mix acetone and ethanol evenly in a ratio of 2:1, then add the prescribed amount of raw materials, povidone K30, and sodium chloride, and stir until completely dissolved;
[0049] 3) Granulation: Add the prescribed amount of sucrose, carboxymethyl starch sodium, and sucralose into the fluidized bed, use the prepared binder for top-spray granulation, and control the inlet air temperature at 60-80°C and the air inlet frequency 30-50HZ, atomization pressure 0.2-0.4Mpa, peristaltic pump speed 20±3rpm;
[0050] 4) Drying: Control the air inlet temperature at 60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com