Process for preparation of esters in aqueous solvent systems
A water-containing solvent and water-solvent technology, which is applied in the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problem of destroying the original configuration of carboxylic acid compounds, affecting product composition and esterification degree, and using catalysts Uncontrollable volume and other problems, to achieve the effect of expanding the range of oil-water partition coefficient, easy control of experimental conditions, and small environmental impact of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
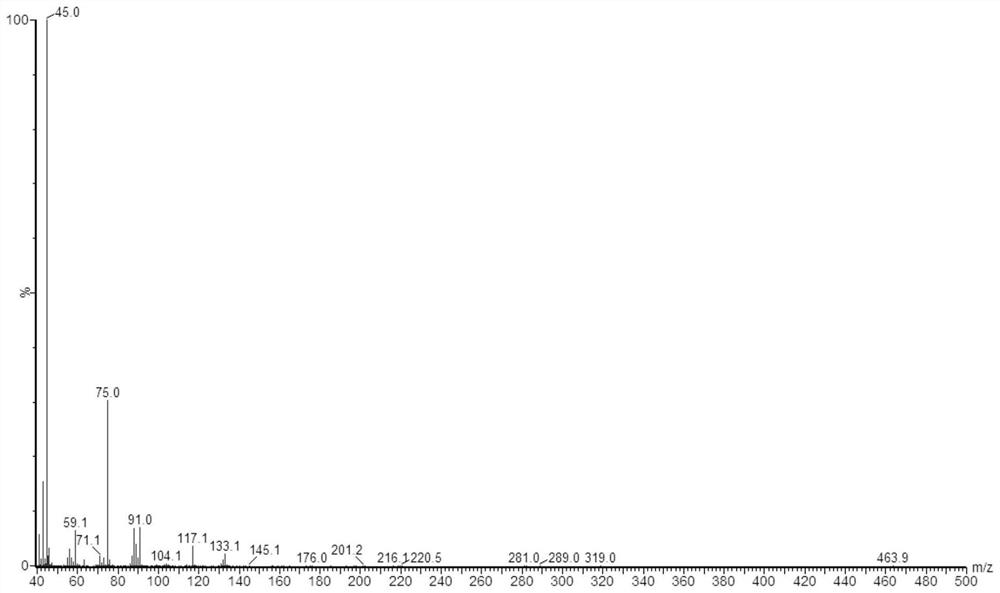
[0054] At room temperature, dissolve 9.008g of lactic acid in 10ml of pure water, and dissolve 26.624g of O-propyl-N,N'-dicyclohexylisourea in 10ml of methanol solution. After fully dissolving, mix the two solutions in 250ml In the spherical reactor, continue to stir at 50°C for 20 hours and then stop the reaction to obtain propyl lactate.
[0055] Samples were taken from the reaction system and diluted with methanol for 10 4 times, tested by gas chromatography tandem time-of-flight mass spectrometer Waters, GCT Premier; the inlet temperature is 280°C, the initial column temperature is 95°C, and then the temperature is raised to 280°C at 15°C / min and kept for 6 minutes. 1 microliter injection. Measure the mass spectrum of propyl lactate, such as figure 1 Shown, and utilize chromatogram peak area to calculate that the productive rate of propyl lactate is 83%.
Embodiment 2
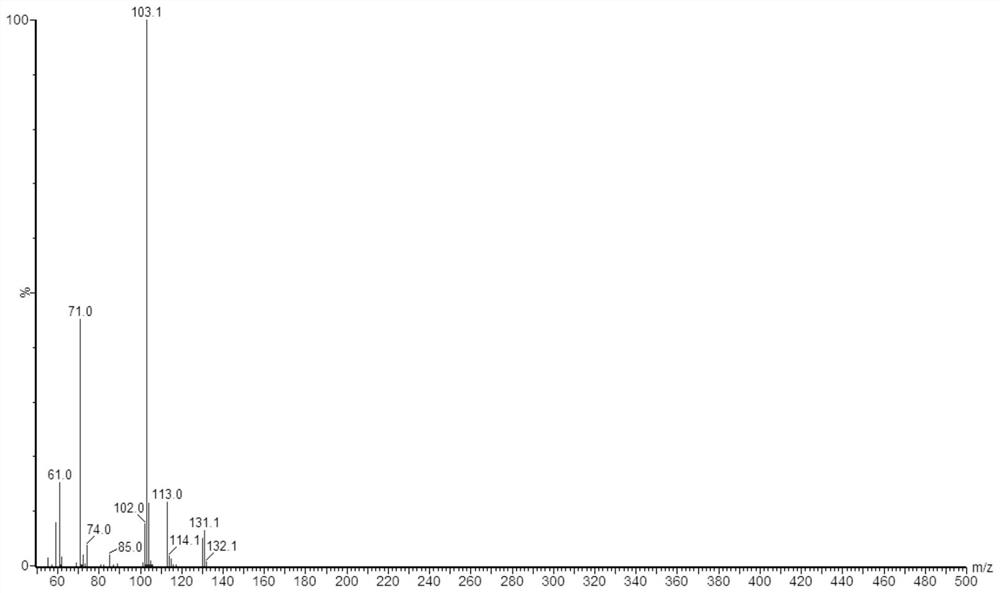
[0057] At room temperature, dissolve 13.409g of malic acid in 20ml of normal saline, and dissolve 23.820g of O-methyl-N,N'-dicyclohexylisourea in 10ml of ethanol solution. After fully dissolving, mix the two solutions in In a 250ml spherical reactor, continue stirring at a constant temperature of 90°C for 24 hours and then stop the reaction.
[0058] Samples were taken from the reaction system and diluted with ethanol for 10 4 Times, by gas chromatography mass spectrometer tandem time-of-flight mass spectrometer Waters, GCT Premier records the mass spectrogram of dimethyl malate, test conditions with reference to embodiment 1, the result is as follows figure 2 Shown, and utilize chromatogram peak area to calculate and find that the productive rate of dimethyl malate is 88%.
Embodiment 3
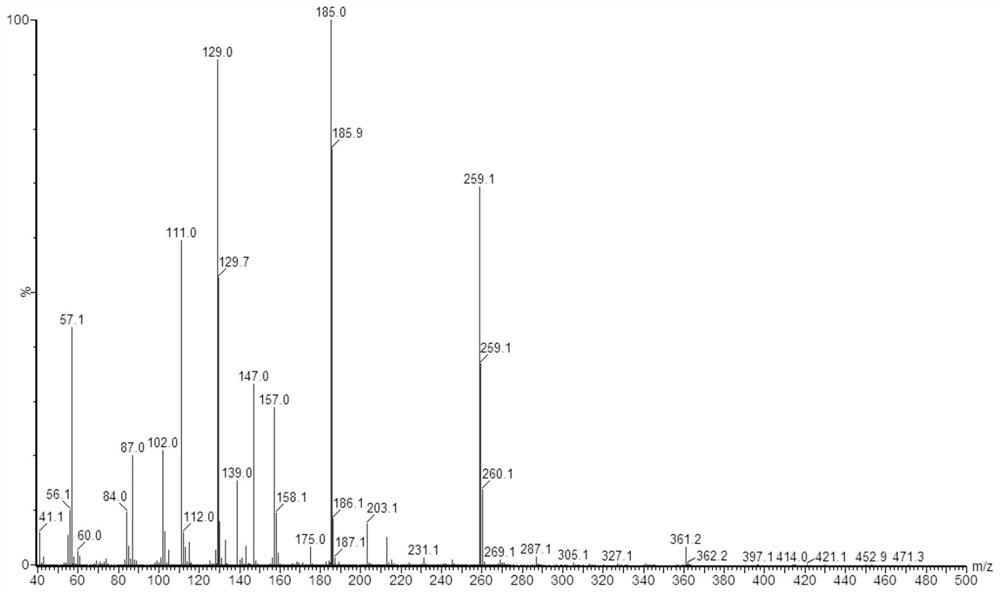
[0060] At room temperature, dissolve 21.014g of citric acid monohydrate in 50ml of water for injection, and dissolve 84.135g of O-butyl-N,N'-dicyclohexylisourea in 50ml of n-butanol solution. After fully dissolving, mix The two solutions were placed in a 250ml spherical reactor, and the reaction was stopped after stirring at a constant temperature of 80°C for 18h.
[0061] Samples were taken from the reaction system and diluted with n-butanol for 10 4 times, by gas chromatography mass spectrometer tandem time-of-flight mass spectrometer Waters, GCT Premier records the mass spectrogram of tributyl citrate, test conditions with reference to embodiment 1, the result is as follows image 3 Shown, and utilize chromatogram peak area to calculate and find that the productive rate of tributyl citrate is 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com