Synthesis method of ergothioneine
A kind of technology of ergothioneine and synthetic method, applied in the field of ergothioneine synthesis, can solve the problems of high cost, low product yield, expensive reagents, etc., and achieve the effect of short synthetic route, high yield and efficient thiolation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A kind of synthetic method of ergothioneine, comprises the following steps:
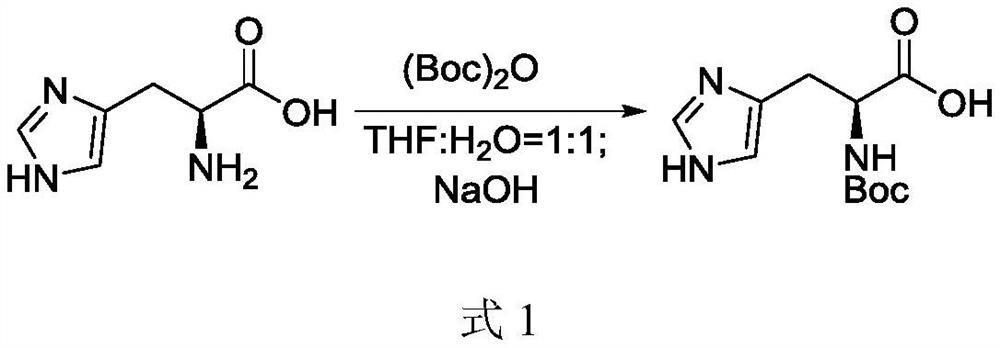
[0029] (1) Boc protection of histidine: dissolve histidine in the first solvent to obtain a histidine solution, add sodium hydroxide and di-tert-butyl dicarbonate in turn, and stir the reaction after adding di-tert-butyl dicarbonate , after the reaction is completed, adjust the pH to 3-5 with formic acid, add water to precipitate a solid, filter and dry to obtain Boc-protected histidine;
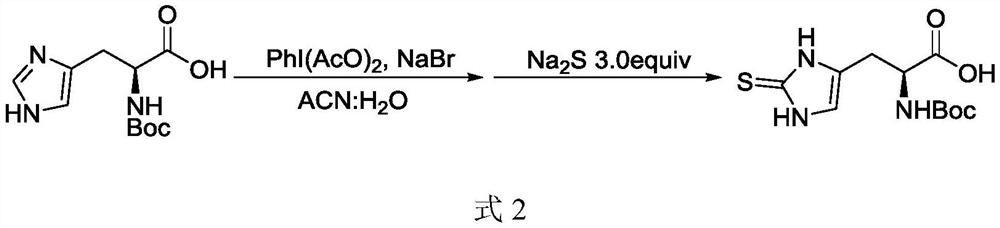
[0030] (2) One-pot thiolation: dissolve the Boc-protected histidine in the second solvent to obtain a Boc-protected histidine solution, add sodium bromide and iodobenzene acetate in turn, stir and react for 2-4 hours, and control the temperature Lower than 40°C, add sodium sulfide after the reaction is completed, raise the temperature to 70°C-100°C for 4-6 hours, after the reaction is completed, cool down to room temperature, add water, then add sulfuric acid to adjust the pH of the solution to 3-5, and prec...
Embodiment 1
[0043] A kind of synthetic method of ergothioneine, comprises the following steps:
[0044] (1) Dissolve histidine (1.55kg, 10mol, 1.0equiv) in 10L of tetrahydrofuran and water in a mixed solvent with a volume ratio of 1:1 at room temperature, and slowly add sodium hydroxide (0.4kg, 10mol, 1.0 equiv), then slowly add di-tert-butyl dicarbonate (2.18kg, 10mol, 1.0equiv), add and stir at room temperature for 10 hours, TLC shows that the reaction is complete, adjust the pH to 4 with formic acid, add 20L of water, and precipitate a large amount of solid , filtered and dried to obtain 2.32 kg of Boc-protected histidine with a yield of 91%.
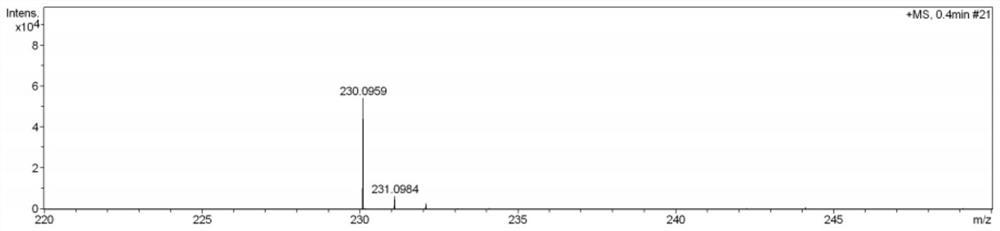
[0045] (2) Dissolve Boc-protected histidine (1.27kg, 5mol, 1.0equiv) in 5L of acetonitrile and water in a mixed solvent with a volume ratio of 1:1 at room temperature, add sodium bromide (0.52kg, 5mol, 1.0equiv), then slowly add iodobenzene acetate (1.61kg, 5mol, 1.0equiv), and control the temperature below 40°C. After the addition was complet...
Embodiment 2
[0050] Same as Example 1, the difference is:
[0051] Step (4) Dissolve thiohistidine (9.3g, 150mM, 1.0equiv) in water at room temperature, slowly add 450mM S-adenosylmethionine (SAM), thiohistidine and S-adenosyl The molar ratio of glycoside methionine is 1:3, then add 0.58 mg EgtD (ergothioneine methyltransferase, derived from Mycobacterium smegmatis) to catalyze the trimethylation reaction of thiohistidine histidine, 37 °C React for 2 hours to generate ergothioneine. After the conversion is complete, use a Sephadex ged column to remove the enzyme. After the product is concentrated under reduced pressure, use a silica gel column to purify to obtain pure ergothioneine. Purified ergothioneine 0.84 g, yield 90.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com