Microneedle for treating psoriasis through percutaneous delivery of lipidosome and preparation method of microneedle
A technology of liposome and psoriasis, which is applied in the field of microneedle and its preparation for the transdermal delivery of liposome for the treatment of psoriasis. Road side effects, improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of MTX liposome
[0050] (1) Prepare liposomes by reverse evaporation method:
[0051] 30 mg of egg yolk lecithin (EPC), 15 mg of cholesterol (Chol) were dissolved in 9 mL of chloroform as an oil phase. Dissolve 6 mg of MTX in 3 mL of PBS with a pH of 6.5, add it to the above oil phase as the water phase, and ultrasonically (power 150 W) with an ice-bath probe for 5 min to form a w / o emulsion. Rotate at 30 °C for 30 min to evaporate the organic solvent to obtain Semi-solid jelly. Continue to add 3 mL of LPBS, and rotate under reduced pressure at 30°C for 5 min to remove residual organic solvent. Finally, in the cell disruptor, the ice-bath probe was ultrasonicated (power 150W) for 5 minutes to obtain a uniform drug-loaded liposome solution.
[0052] (2) MTX liposome lyophilized powder preparation:
[0053] After the liposome solution was dialyzed (3500Da, 48h), the free drug MTX was removed, and the lyoprotectant (trehalose) was added t...
Embodiment 2
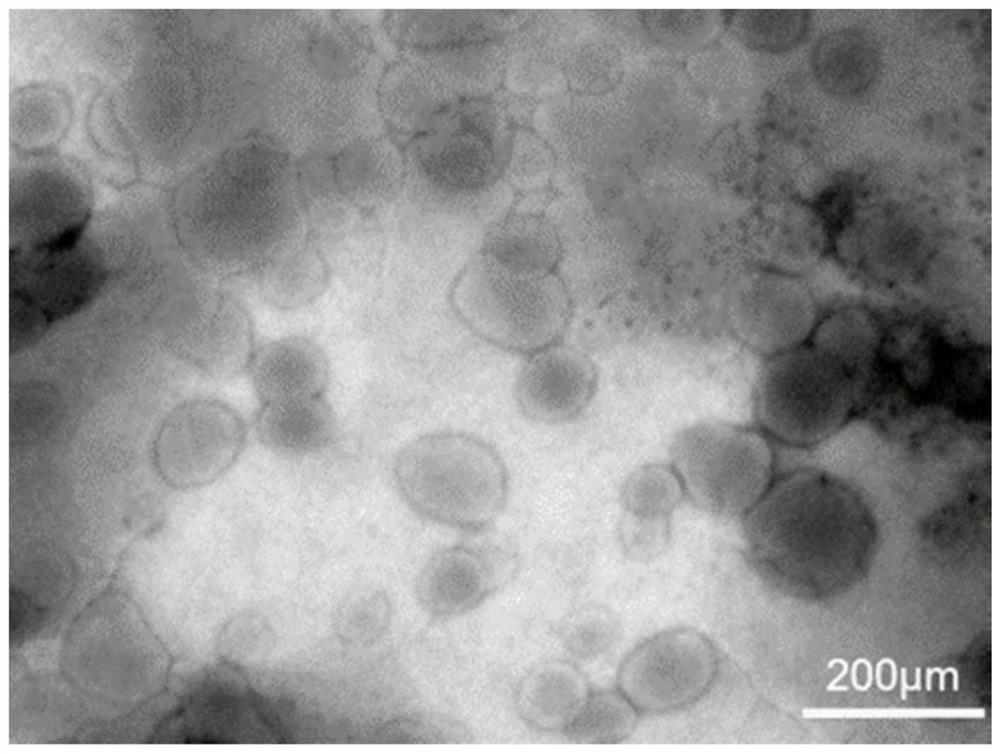
[0054] Embodiment 2: Characterization of MTX liposomes
[0055] (1) Measurement of liposome particle size, PDI, zeta potential:
[0056] The particle size, PDI and zeta potential of the liposomes prepared in Example 1 were measured at 25° C. with a DLS particle size analyzer. Add the MTX liposome solution into the particle size cup, and measure in parallel three times. During the test, record the average hydrodynamic diameter and the polydispersity index (PDI), add a potentiometric electrode, make the electrode piece completely immersed in the solution to be tested, measure in parallel three times, record the zeta potential of the liposome during the test, and analyze .
[0057] Precisely draw 1mL of liposomes into a 100mL test tube, add 5mL of demulsifier (chloroform / methanol = 1:1 (V / V)), mix well, and ultrasonicate for 30-40min to make the demulsification uniform, after standing still, take Dilute the upper layer solution, measure its absorbance value at 303nm with a UV ...
Embodiment 3
[0070] Example 3: Preparation of blank soluble microneedles
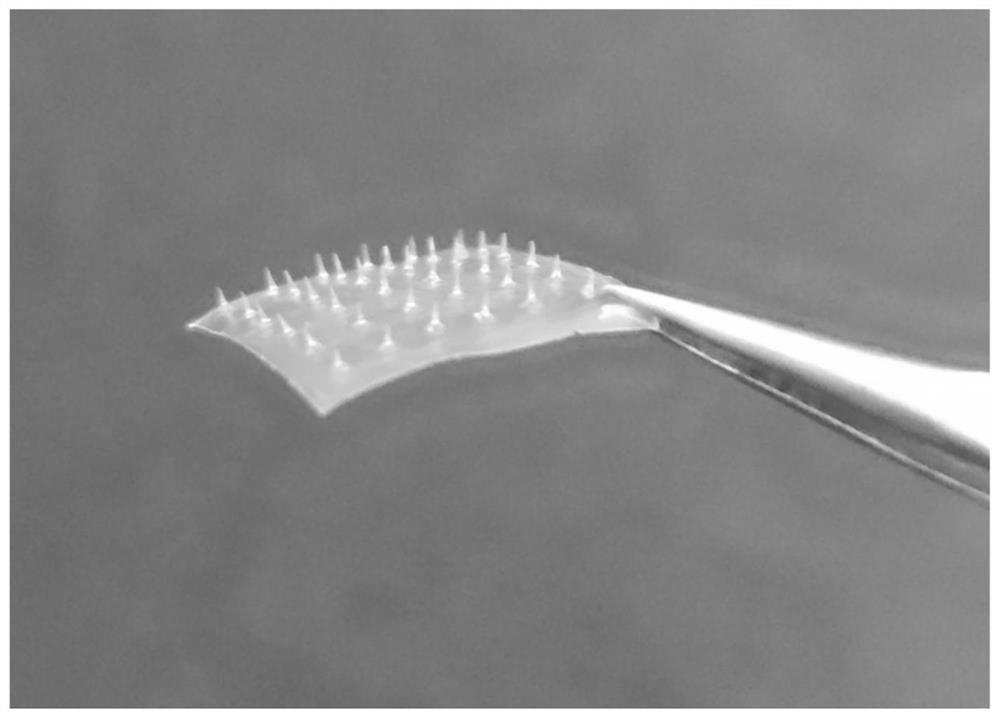
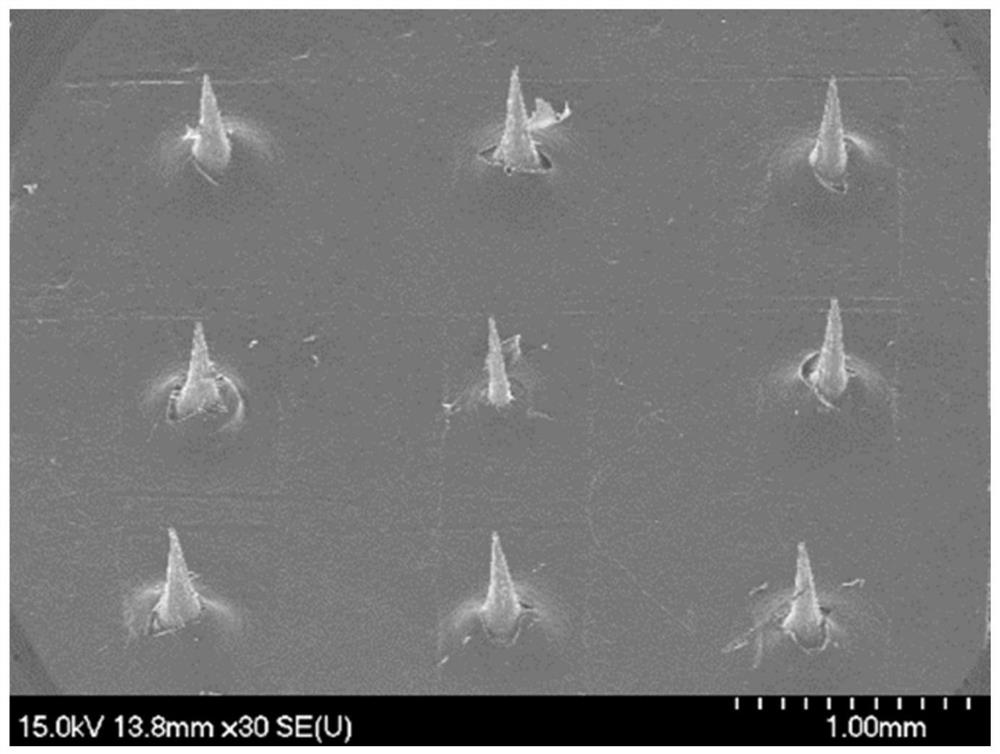
[0071] The microneedle mold is made of polydimethylsiloxane PDMS, which can be made figure 1 The 6×6 conical MNs shown (the bottom diameter of a single MNs is 130 μm, the tip diameter is 12 μm, and the needle length is 500 μm).
[0072] The soluble microneedle is prepared according to the following steps: weigh methyl vinyl ether-maleic acid PMVE MAH, add purified water to dissolve, and mix well on a four-dimensional rotary mixer to obtain PMVE MAH solution, which is slightly yellow. Place PMVE MAH in a microneedle mold made of polydimethylsiloxane PDMS, centrifuge at 3500rad / min for 30min to form needles; scrape off excess needle material PMVE MAH, and place the mold in a vacuum desiccator at room temperature , after negative pressure drying for 12 hours, the mixed backing material of HPC and WCS was centrifuged for 30 minutes at a reduced speed (horizontally centrifuged for 5 minutes each at 3000, 2500, 2000, 150...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Tip diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com