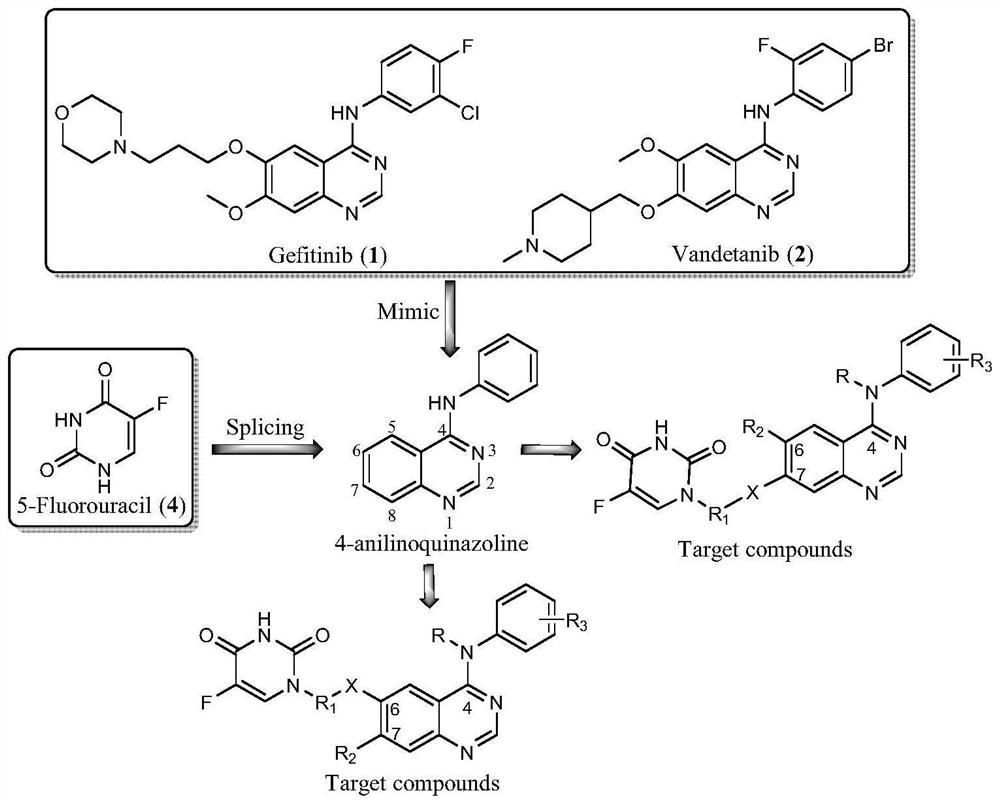
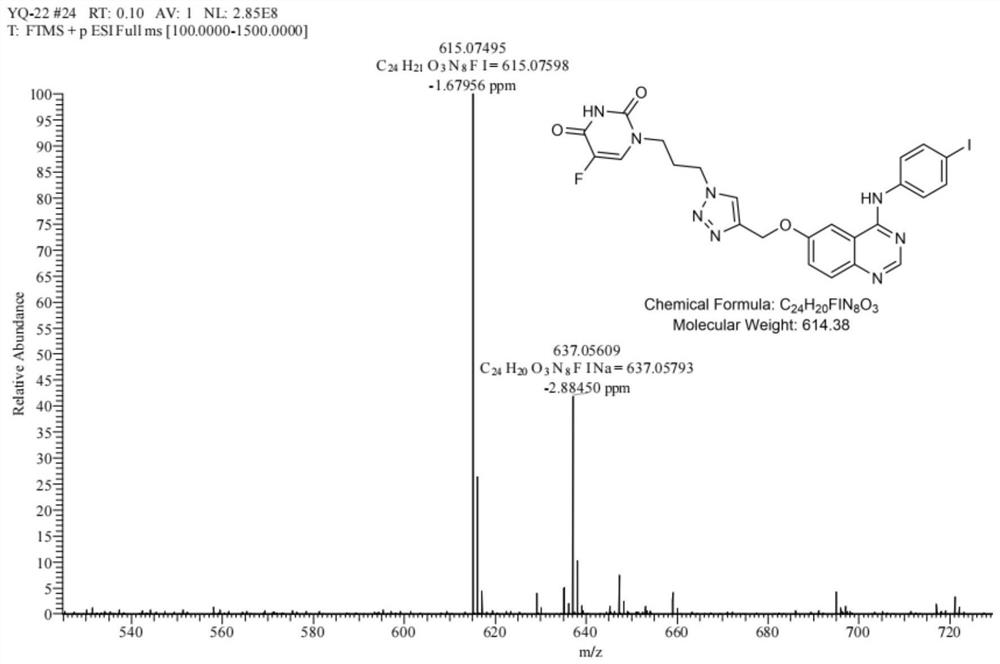
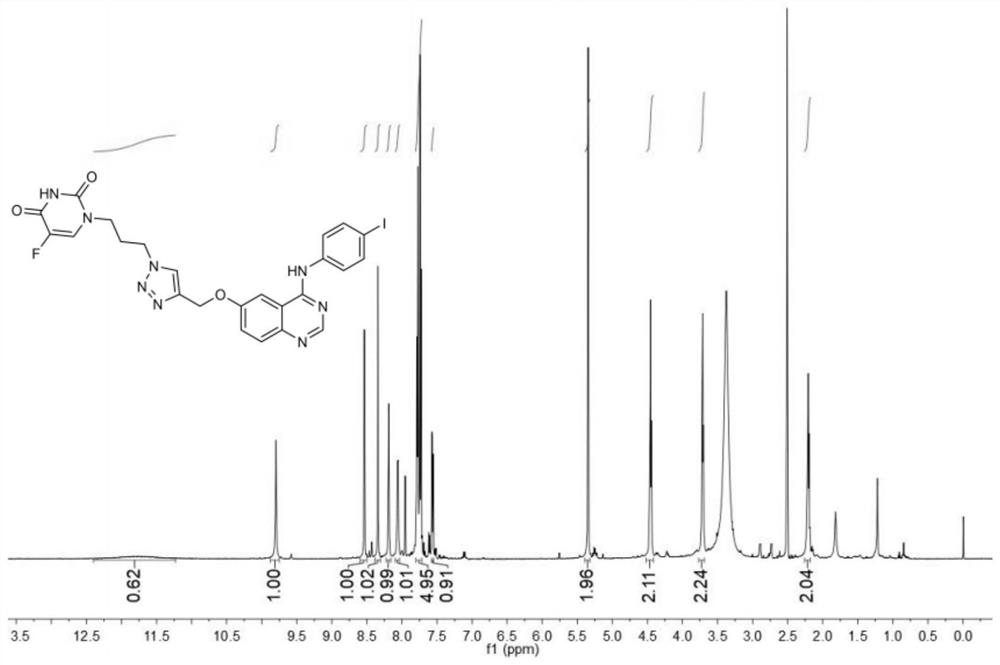
5-fluorouracil spliced 4-aniline quinazoline compound as well as preparation method and application thereof
A technology of aniline quinazoline and fluorouracil, applied in the field of chemistry, can solve the problems of drug resistance mutation, inability to effectively kill cancer cells, drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The chemical synthesis of 5-fluorouracil splicing 4-aniline quinazoline compounds (E1) with formula (I), the preparation methods are shown in Table 1, the preparation methods of compounds E2~E3 are exactly the same, but it should be emphasized that the compounds of the present invention are not limited to those shown in Table 1.
[0034] Table 1 has the chemical synthesis of formula (I) 5-fluorouracil splicing 4-aniline quinazoline compounds
[0035]
[0036] Take the 5-fluorouracil alkyl intermediate (A, 6.0g, 23.9mmol) in a 250mL reaction flask, add 50mL of anhydrous tetrahydrofuran and stir to dissolve, then add azidotrimethylsilane (13.5mL, 95.6mmol, 4.0eq. ) and 1mol / L tetrabutylammonium fluoride tetrahydrofuran solution (95.6mL, 95.6mmol, 4.0eq.). Reaction in an oil bath at 65°C, monitored by thin-layer chromatography (TLC), and the reaction was complete in about 20 hours. The reaction solution was diluted with 400 mL of ethyl acetate, then extracted with 300 ...
Embodiment 2
[0041] The chemical synthesis of 5-fluorouracil splicing 4-aniline quinazoline compound (G1) with formula (I), the preparation method is shown in Table 2, and the preparation method of compound G2 is exactly the same, but it should be emphasized that the compound of the present invention is not limited to Table 2 2 represents the content.
[0042] Table 2 has the chemical synthesis of formula (I) 5-fluorouracil splicing 4-aniline quinazoline compounds
[0043]
[0044] Take 4-anilinoquinazoline compound F1 (100mg, 0.34mmol), compound E (78mg, 0.41mmol, 1.2eq.) in a 25mL eggplant-shaped bottle, add N,N-dimethylformamide 10mL, stir to make it Dissolve completely, add DCC (142mg, 0.69mmol, 2.0eq.), DMAP (84mg, 0.69mmol, 2.0eq.), EDCI (198mg, 1.03mmol, 3.0eq.) successively, continue to stir the reaction at room temperature, thin layer chromatography ( TLC) monitoring, the reaction was complete in about 4 days, the reaction solution was diluted with 40mL ethyl acetate, then ext...
Embodiment 3
[0047]The chemical synthesis of 5-fluorouracil splicing 4-aniline quinazoline compound (J) with formula (II) is shown in Table 3 for the preparation method, but it should be emphasized that the compounds of the present invention are not limited to the content shown in Table 3.
[0048] Table 3 has the chemical synthesis of formula (II) 5-fluorouracil splicing 4-aniline quinazoline compounds
[0049]
[0050] Take 4-anilinequinazoline compound H (50mg, 0.11mmol), compound B (23mg, 0.11mmol, 1.0eq.) in a 25mL eggplant-shaped bottle, add 5mL of N,N-dimethylformamide, stir to make it Completely dissolved, under the condition of avoiding light, add sodium ascorbate (21mg, 0.11mmol, 1.0eq.), cuprous iodide (8mg, 0.04mmol, 0.4eq.), N,N-diisopropylethylamine (17mg , 0.13mmol, 1.2eq.), continue to stir the reaction at room temperature, monitor by thin layer chromatography (TLC), the reaction is complete in about 15 hours, the reaction solution is diluted with 40mL ethyl acetate, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com