Continuous flow efficient production method of 2-amino-4-nitrotoluene
A technology of nitrotoluene and production methods, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of reducing the risk factor of the reaction, unfavorable treatment of large amount of waste acid, and long reaction cycle, etc., to achieve Good fluidity, improved atom economy, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
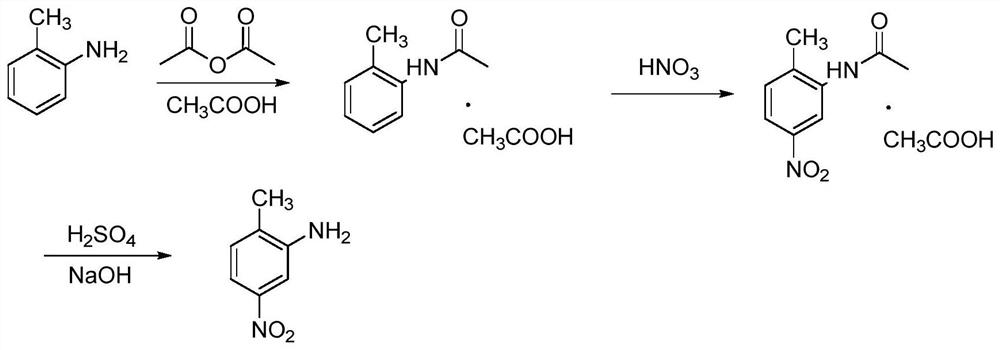
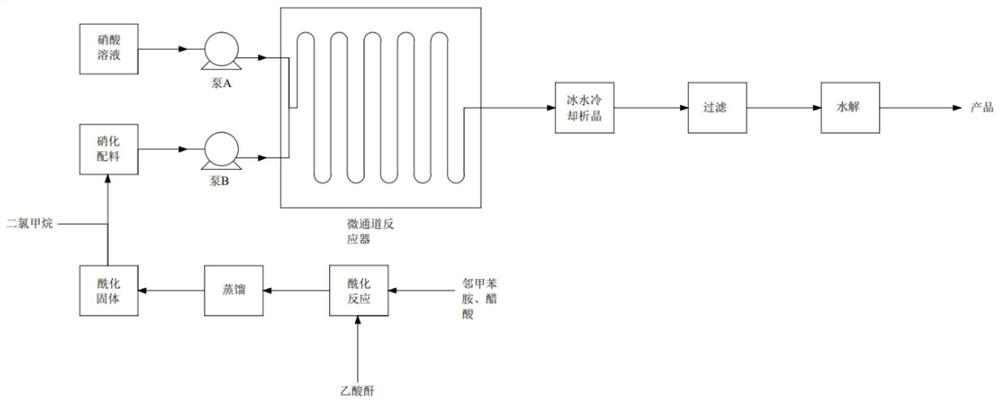
[0032] (1) Dissolve 1 mol o-toluidine in 6 mol acetic acid, add 1 mol acetic anhydride dropwise in a stirred tank reactor for acylation reaction, control the acylation reaction temperature at 20°C, and add acetic anhydride dropwise for 1 hour , and concentrated by distillation to obtain o-methylacetanilide acetate;
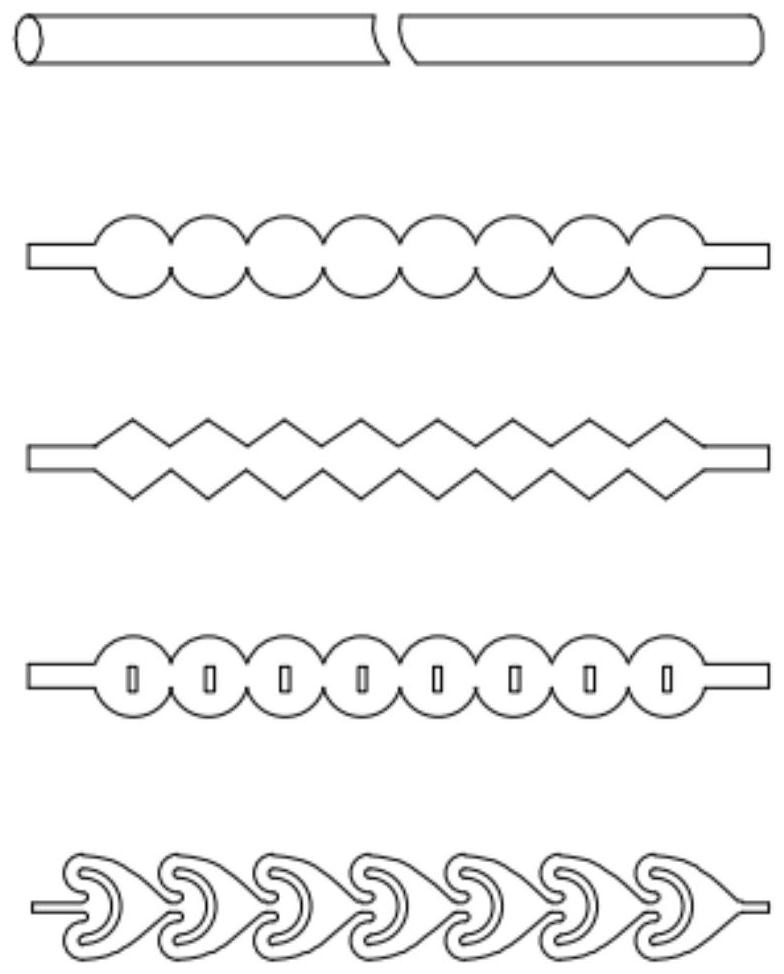
[0033] (2) o-methylacetanilide acetate is dissolved in dichloromethane solvent and the 50% nitric acid solution that configures, according to o-methylacetanilide acetate / nitric acid molar ratio 1:1 feed, pump respectively Into a microchannel reactor module with a direct-flow channel and a circular cake-type pulse variable-diameter rectangular flat pipe for nitration reaction, the reaction temperature is controlled at 20°C, the residence time is 1min, and the pressure is 1bar, and the nitration liquid is collected at the end of the reactor. Dissolved in ice water, crystallized, and separated by filtration to obtain m-nitro-o-methylacetanilide acetate, and the filtr...
Embodiment 2
[0036] (1) Dissolve 1 mol of o-toluidine in 6.1 mol of acetic acid, add 1.03 mol of acetic anhydride dropwise in a stirred tank reactor for acylation reaction, control the temperature of the acylation reaction at 21°C, and the time for adding acetic anhydride For 1.1h, concentrated by distillation to obtain o-methylacetanilide acetate;
[0037](2) o-methylacetanilide acetate is dissolved in dichloroethane solvent and the 55% nitric acid solution that configures, feeds according to o-methylacetanilide acetate / nitric acid molar ratio 1:1.05, respectively Pumped into the microchannel reactor module with the structure of straight-through channel and oblique square cake pulse variable diameter rectangular flat pipe for nitrification reaction, the reaction temperature is controlled at 25°C, the residence time is 1.5min, the pressure is 2bar, and it is collected at the end of the reactor The nitration solution is dissolved in ice water, crystallized, filtered and separated to obtain ...
Embodiment 3
[0040] (1) Dissolve 1 mol of o-toluidine in 6.2 mol of acetic acid, add 1.05 mol of acetic anhydride dropwise in a stirred tank reactor for acylation reaction, control the temperature of the acylation reaction at 22°C, and the time for adding acetic anhydride For 1.2h, concentrated by distillation to obtain o-methylacetanilide acetate;
[0041] (2) Dissolve o-methylacetanilide acetate in chloroform solvent and the prepared 60% nitric acid solution, feed the material according to the o-methylacetanilide acetate / nitric acid molar ratio of 1:1.1, and pump them into the structure respectively The nitrification reaction is carried out in the microchannel reactor module of the direct-flow channel and the enhanced mixed round cake rectangular flat pipe, the reaction temperature is controlled at 30°C, the residence time is 2min, and the pressure is 3bar. The nitrification liquid is collected at the end of the reactor and passed through ice water. Dissolving, crystallizing, filtering a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com