Stilbene derivative as well as preparation and application thereof
A technology of stilbene and derivatives, which is applied in the field of preparation of luminescent organic substances, can solve problems such as low solubility and high fluorescence quenching effect, and achieve the effect of reducing fluorescence quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] Preparation of 4-(1-adamantyl)bromobenzene:
[0049] Add adamantanol (2.0g, 13.14mmol) and bromobenzene (2.0631g, 13.14mmol, 1eq) into a 250ml two-necked flask, dissolve in 80ml of dry dichloromethane, and slowly add trifluoroethylene dropwise at -5°C under nitrogen protection. Methanesulfonic acid (5.9162g, 39.42mmol, 3eq). React for 3 hours. Raise to 25°C, add saturated aqueous sodium carbonate solution until the reaction solution is neutral. The organic phase was extracted and separated, and the organic phase was washed twice with saturated brine. The organic phase was concentrated to dryness under reduced pressure and purified by column chromatography (n-hexane) to obtain 2.87 g of 4-(1-adamantane)bromobenzene (S1), with a yield of 75%. The physical property data obtained by proton nuclear magnetic resonance spectrum are as follows: 1 H NMR (400MHz, CDCl 3): δ=7.42(dt, J=8.68, 2.76Hz, 2H), 7.23(dt, J=8.64, 2.68Hz, 2H), 2.09(s, 3H), 1.87(d, J=2.6Hz, 6H) ,1.76(d...
Embodiment 1
[0057] Preparation of 4,4'-bis(4-(3-(1-adamantyl)-9carbazolyl)styryl)-1,1'-biphenyl (target product):
[0058] 4-[3-(1-adamantyl)-9H-carbazol-9-yl]benzaldehyde (S4) (1.4g, 3.452mmol) and 4,4'-bis(diethoxyphosphonoform Base) biphenyl (0.6289g, 1.381mmol, 1eq) was mixed into a 250ml two-necked flask, 100ml of tetrahydrofuran was added, potassium tert-butoxide (0.7747g, 6.904mmol, 5eq) was added in batches, and reacted for 3h. The reaction solution was concentrated under reduced pressure, the obtained product was washed twice with water and twice with methanol, and the product was purified by recrystallization (toluene) to obtain 0.95 g of 4,4'-bis(4-(3-(1-adamantyl)-9carba Azolyl) styryl) -1,1'-biphenyl (a-BSBCz), yield 71.8%. The physical property data obtained by proton nuclear magnetic resonance spectrum are as follows: 1 H NMR (400MHz, CD 2 Cl 2 ): δ=8.20(d, J=7.84Hz, 2H), 8.17(d, J=1.6Hz, 2H), 7.84(d, J=8.48Hz, 4H), 7.75(dd, J=5.68, 8.52Hz ,8H),7.64(d,J=8.40Hz,4H),7.56...
Embodiment 2
[0060] Film preparation of 4,4'-bis(4-(3-(1-adamantyl)-9carbazolyl)styryl)-1,1'-biphenyl:
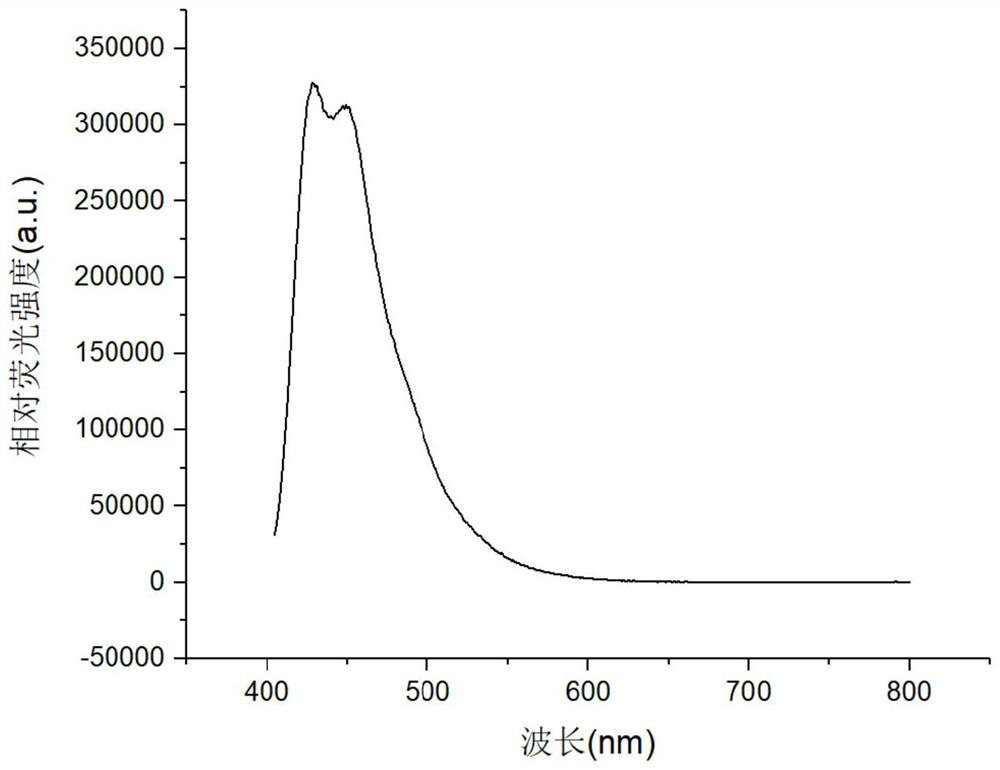
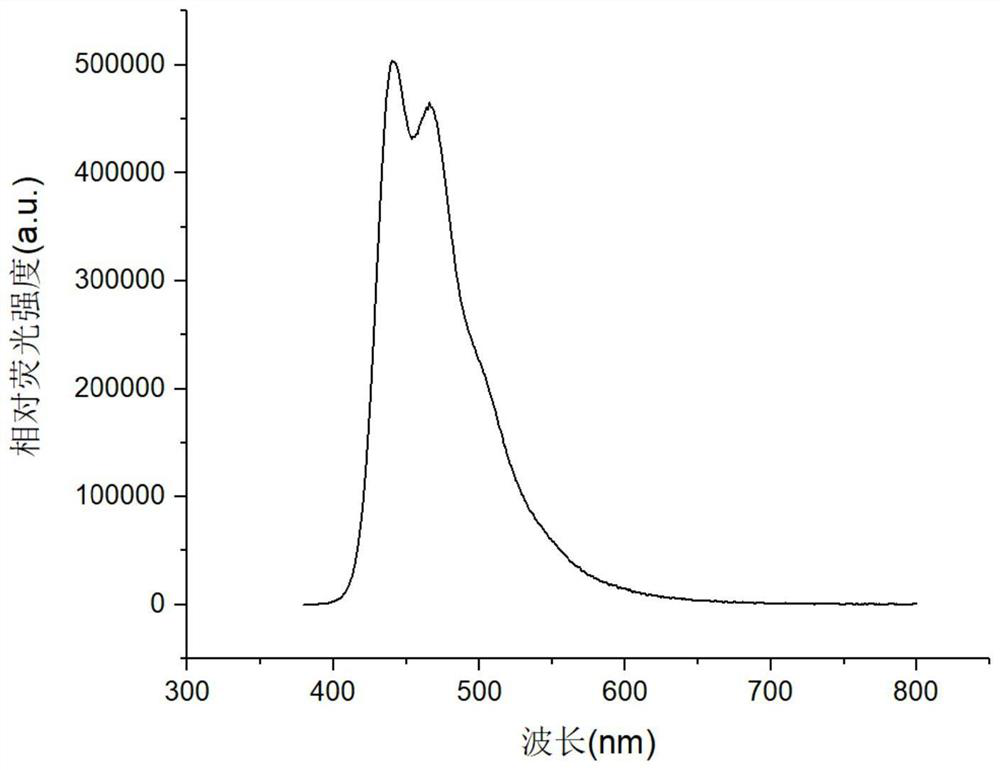
[0061] Put 4,4'-bis(4-(3-(1-adamantyl)-9carbazolyl)styryl)-1,1'-biphenyl in a quartz crucible and put it into a coating machine for evaporation In the coil, select the substrate, use deionized water, acetone, and isopropanol as solvents, ultrasonically for 15 minutes, blow dry with a nitrogen gun, and ultraviolet ozone for 20 minutes, and put it into the substrate bottom plate of the coating machine. The pressure in the inner cavity of the coating machine is pumped below 5×10-4Pa, and the average evaporation rate can be monitored to be 0.6 Evaporate to a thickness of 120nm to obtain a pure film of 4,4'-bis(4-(3-(1-adamantyl)-9carbazolyl)styryl)-1,1'-biphenyl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com