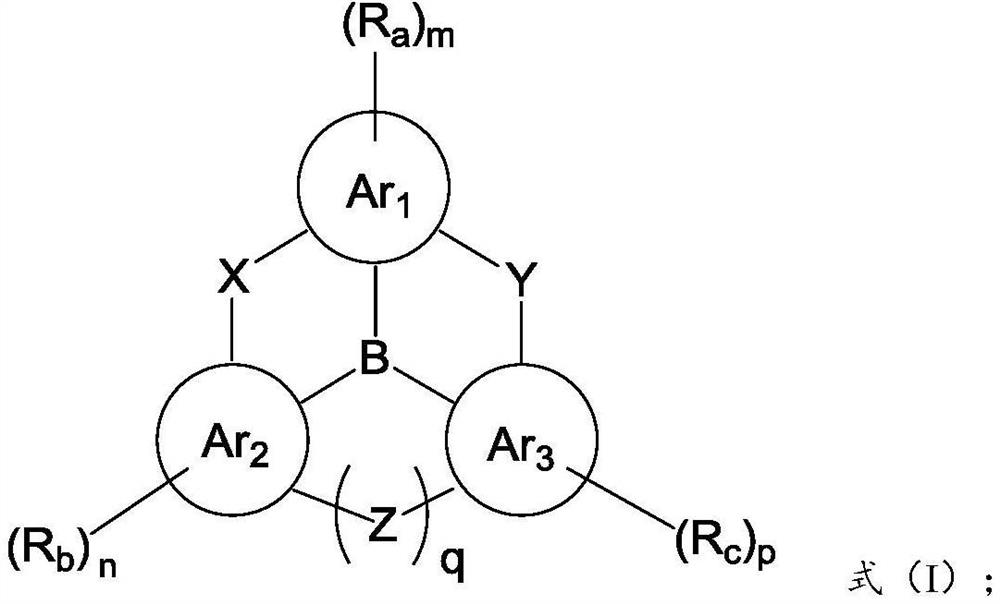
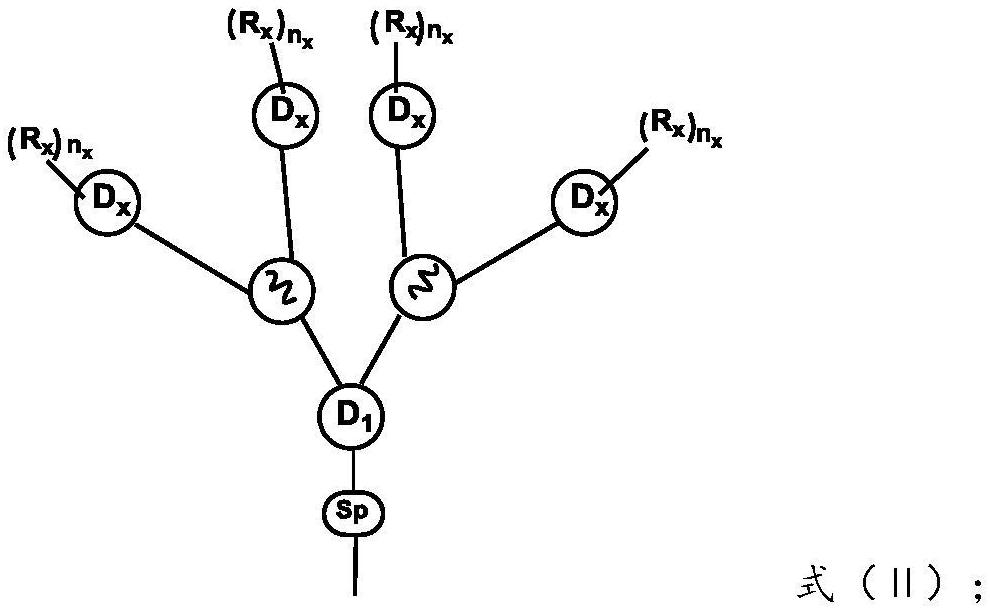
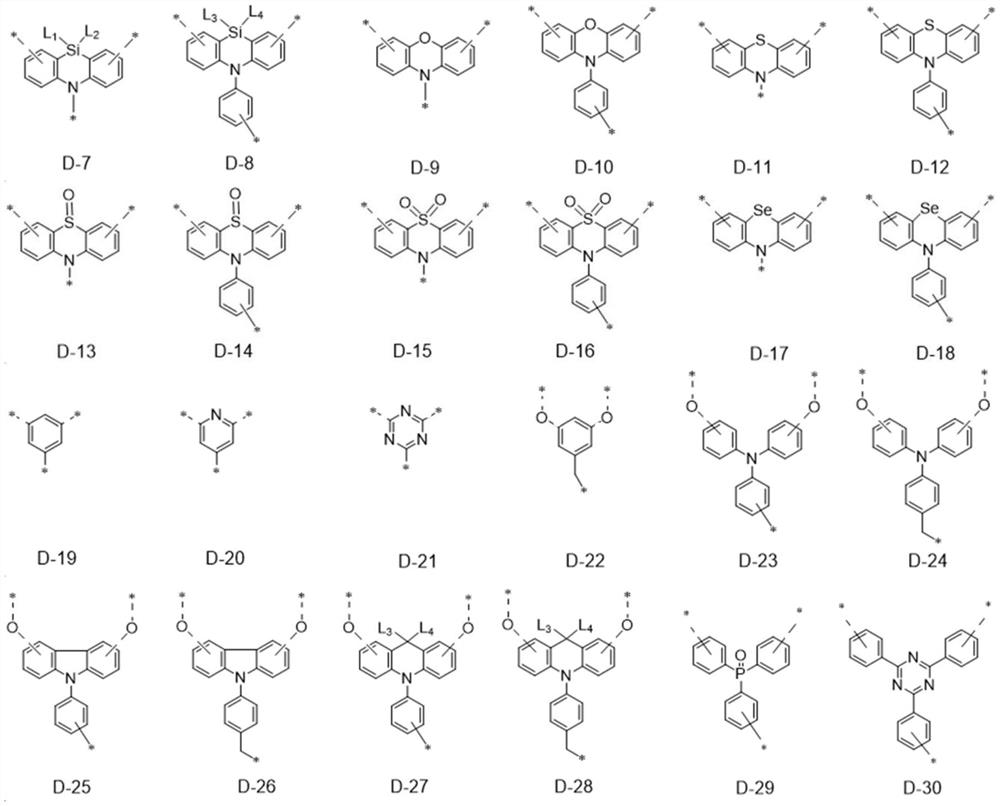
Dendritic fused ring compound containing boron atoms and oxygen atoms, preparation method and application thereof and organic electroluminescent device
A technology of dendritic and boron atoms, applied in the field of dendritic condensed ring compounds and organic electroluminescent devices, can solve the problems of low color purity and wide emission spectrum.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Example 1: Preparation of compounds of formula I-1
[0154] The synthetic route and process are as follows:
[0155]
[0156] Under an argon atmosphere, in a 500 mL three-necked flask, weigh the compound of formula 1-1 (13.6 g, 0.05 mol), phenol (4.7 g, 0.05 mol), thiophenol (5.5 g, 0.05 mol) and K 2 CO 3(13.8g, 0.10mol), add 80mL N-methylpyrrolidone (NMP) into the bottle, heat up to 150°C, stir and react under argon protection for 10 hours, then cool to room temperature, dilute the reaction solution with toluene, pour it out Pour into water, separate the organic phase, add anhydrous sodium sulfate to dry, remove the solvent from the organic phase obtained by filtration, and separate the crude product column to obtain product 1-2 (8.7 g, yield: 40%). Elemental Analysis: Theoretical C, 49.57; H, 2.77; S, 7.35; Tested C, 49.67; H, 2.97; S, 7.15. Electrospray ionization mass spectrometry (ESI-MS): theoretical value 433.9, experimental value 433.9 (M + ).
[0157] U...
Embodiment 2
[0161] Example 2: Preparation of compounds of formula 1-5
[0162] The synthetic route and process are as follows:
[0163]
[0164] Under argon atmosphere, in a 500mL three-necked flask, weigh the compound of formula 1-1 (13.6g, 0.05mol), 4-pyridinethiol (11.1g, 0.10mol) and K 2 CO 3 (13.8g, 0.10mol), add 80mL N-methylpyrrolidone (NMP) into the bottle, heat up to 150°C, stir and react under argon protection for 10 hours, then cool to room temperature, dilute the reaction solution with toluene, pour it out Pour into water, separate the organic phase, add anhydrous sodium sulfate to dry, remove the solvent from the organic phase obtained by filtration, and separate the crude product column to obtain product 2-2 (9.1 g, yield: 40%). Elemental analysis: theoretical C, 42.31; H, 2.22; N, 6.17; S, 14.12; found C, 42.41; H, 2.32; N, 6.11; S, 14.11. ESI-MS: theoretical value 451.8, experimental value 452.9 ([M+H] + ).
[0165] Under argon atmosphere, the compound of formula 2...
Embodiment 3
[0168] Example 3: Preparation of compounds of formula I-11
[0169] The synthetic route and process are as follows:
[0170]
[0171] Under an argon atmosphere, weigh the compound of formula 1-1 (13.6 g, 0.05 mol), 4-hydroxy-9,9'-dimethylfluorene (21.0 g, 0.10 mol) and K in a 500 mL three-necked flask 2 CO 3 (13.8g, 0.10mol), add 80mL N-methylpyrrolidone (NMP) into the bottle, heat up to 150°C, stir and react under argon protection for 10 hours, then cool to room temperature, dilute the reaction solution with toluene, pour it out Pour into water, separate the organic phase, add anhydrous sodium sulfate to dry, remove the solvent from the organic phase obtained by filtration, and obtain the product 3-2 (13.0 g, yield: 40%) by column separation of the crude product. Elemental Analysis: Theoretical C, 66.27; H, 4.33; Tested C, 66.31; H, 4.45. ESI-MS: theoretical value 650.0; experimental value 651.0 ([M+H] + ).
[0172] Under an argon atmosphere, the compound of formula 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com