Liquid phase detection method for detecting p-chlorobenzoyl chloride
A technology of p-chlorobenzoyl chloride and a detection method, which is applied in the field of drug analysis, can solve the problems of large detection error, large chromatographic column damage, large detection result error, etc., and achieves high system applicability, good linear relationship, and good accuracy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Experimental materials and instrument conditions
[0045] Instrument: Thermo Fisher Ultimate 3000, chromatographic column: Agilent InfinityLab Poroshell 120PFP 4.6×250mm, 4μm; flow rate: 1.0mL / min; column temperature: 10℃; injection volume: 5μL, detection wavelength: 200nm; mobile phase A: Phosphate buffer (20 mM potassium dihydrogen phosphate aqueous solution, adjusted to pH 2.0 with phosphoric acid, potassium dihydrogen phosphate is AR grade, phosphoric acid is HPLC grade, water is high-purity water): acetonitrile (acetonitrile is HPLC grade) = 75:25 (v / v), mobile phase B: acetonitrile (acetonitrile is HPLC grade), the gradient elution procedure is shown in Table 2.
[0046] Table 2. Gradient elution procedure
[0047] time (min) 0 15 35 60 60.1 70 Mobile phase A (%, v / v) 100 100 80 43 100 100 Mobile phase B (%, v / v) 0 0 20 57 0 0
[0048] (2) Experimental steps (take m-chlorobenzoyl chloride as an example)
[0049] Pr...
Embodiment 2
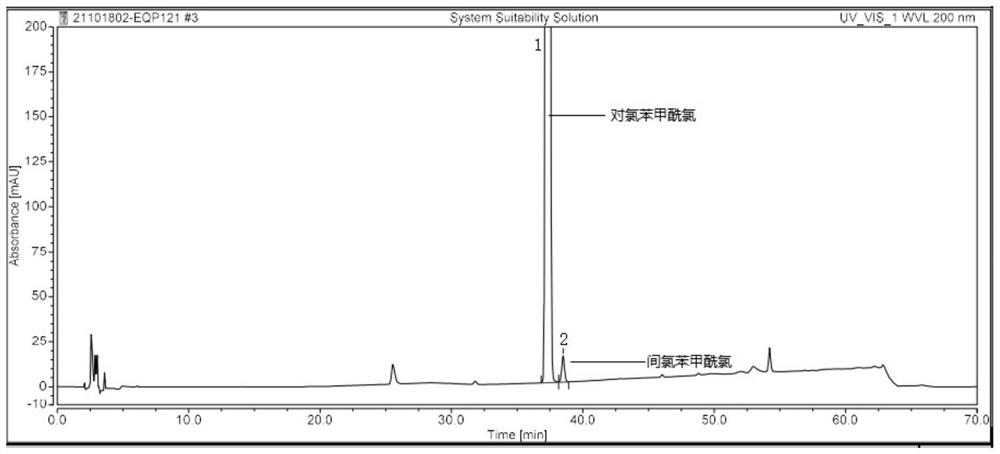
[0078] Example 2: System suitability test of the detection method of the present invention
[0079] The system suitability is achieved by the system suitability solution and the 6-pair control solution. It is required that the resolution between the chromatographic peaks of p-chlorobenzoyl chloride and m-chlorobenzoyl chloride in the system suitability solution is not less than 1.5, and the number of theoretical plates is equal to the number of pairs. Chlorobenzoyl chloride chromatographic peak count, not less than 5000; the RSD of the chromatographic peak area of p-chlorobenzoyl chloride in 6 needles of the control solution is not greater than 5.0%, and the RSD of the retention time is not greater than 1.0%; 7 pairs of p-chlorobenzene in the control solution The RSD of the chromatographic peak area of formyl chloride is not more than 5.0%, and the RSD of the retention time is not more than 1.0%.
Embodiment 3
[0080] Example 3: Specificity of the detection method of the present invention
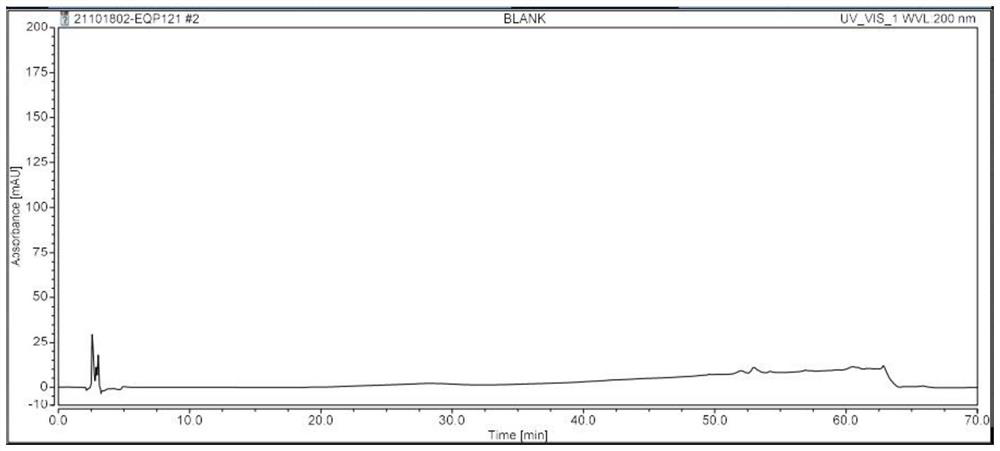
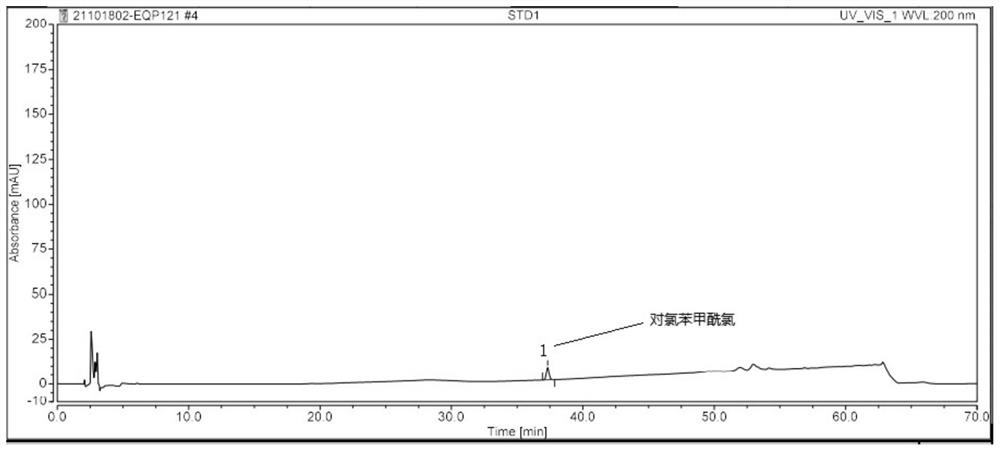
[0081] Specificity is achieved by assaying blank solutions, positioning solutions, test solutions, and spiked test solutions. It is required that the blank solution has no interference with the detection of related substances; the peak purity of the chromatographic peaks in the positioning solution is not less than 990; the detection of related substances in the test solution has no interference, the separation degree between the chromatographic peaks is not less than 1.5, and the peaks of the chromatographic peaks are not less than 1.5. The purity is not less than 990; the detection of related substances in the spiked test solution has no interference, the resolution between chromatographic peaks is not less than 1.5, and the peak purity of the chromatographic peaks is not less than 990.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com