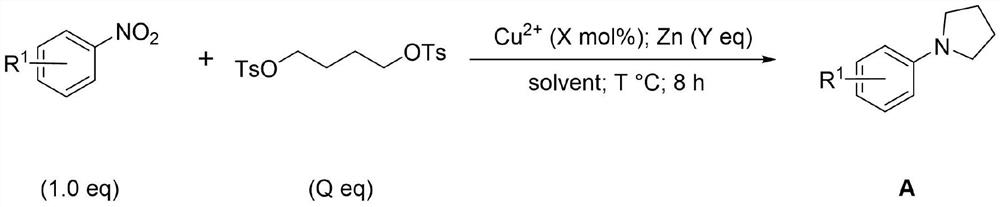
Synthesis method of 1-phenylpyrrolidine
A technology of phenylpyrrolidine and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of complex synthesis of nanoparticles, very harsh reaction temperature requirements, metal cobalt toxicity, etc., achieve the effect of low experimental operation level and broaden the synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
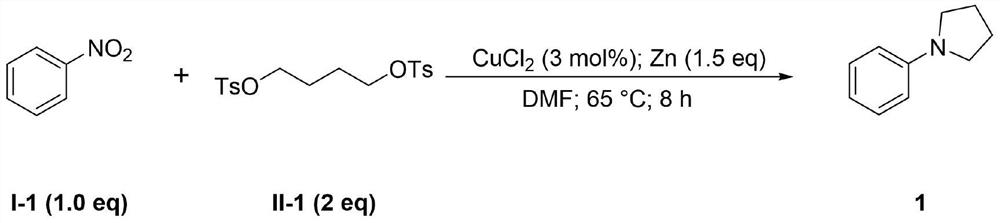
[0035] The synthesis of the present embodiment 1-phenylpyrrolidine 1 is:
[0036]
[0037]Under nitrogen atmosphere, copper(II) chloride [2 mg, 0.015 mmol], zinc powder (49 mg, 0.75 mmol), solvent DMF (2 mL), nitrobenzene (51 μL, 0.5 mmol), and nitrobenzene (51 μL, 0.5 mmol) were successively added to a dry 25 mL schlenk reaction tube. ), 1,4-butanediylbis(4-methylbenzenesulfonate) (321 μL, 1 mmol). The reaction was carried out on a heater with a rotating speed of 360 rpm and a temperature of 65 °C for 8 hours. The reaction of the raw materials was detected by TLC, and the reaction was stopped. The reaction mixture was extracted several times with a mixture of distilled water and ethyl acetate with a volume ratio of 1:2. phase, and dried over anhydrous magnesium sulfate, the organic phase was concentrated, and separated by column chromatography with the eluent (ethyl acetate / petroleum ether=50 / 1) to obtain 67 mg of a light yellow liquid with a yield of 94%.
[0038] 1 H N...
Embodiment 2
[0040] The synthesis of present embodiment 1-(p-tolyl)pyrrolidine 2 is:
[0041]
[0042] Under nitrogen atmosphere, copper(II) sulfate [4 mg, 0.025 mmol], zinc powder (49 mg, 0.75 mmol), solvent Toluene (0.5 mL), p-nitrotoluene (69 mg, 0.5 mmol), 1,4-butanediylbis(4-methylbenzenesulfonate) (468 μL, 1.5 mmol). The reaction was carried out on a heater with a rotating speed of 200 rpm and a temperature of 50 °C for 8 hours. The reaction of the raw materials was detected by TLC and the reaction was stopped. The reaction mixture was extracted several times with a mixture of distilled water and ethyl acetate with a volume ratio of 1:2. phase, and dried over anhydrous magnesium sulfate, the organic phase was concentrated, and then subjected to column chromatography with the eluent (ethyl acetate / petroleum ether=50 / 1) to obtain 76 mg of a light yellow liquid with a yield of 94%.
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.01(d,2H),6.54(d,2H),3.25(t,4H),2.27(s,3H),2.05–1.94(m,4H). 13 ...
Embodiment 3
[0045] The synthesis of the present embodiment 1-(4-fluorophenyl)pyrrolidine 3 is:
[0046]
[0047] Under a nitrogen atmosphere, to a dry 25 mL schlenk reaction flask were successively added copper(II) oxalate [3.8 mg, 0.025 mmol], zinc powder (49 mg, 0.75 mmol), solvent NMP (2 mL), 4-fluoronitrobenzene ( 70.5 mg, 0.5 mmol), 1,4-butanediylbis(4-methylbenzenesulfonate) (468 μL, 1.5 mmol). The reaction was carried out on a heater with a rotating speed of 150 rpm and a temperature of 80 °C for 8 hours. The reaction of the raw materials was detected by TLC, and the reaction was stopped. The reaction mixture was extracted several times with a mixture of distilled water and ethyl acetate with a volume ratio of 1:2. phase, and dried over anhydrous magnesium sulfate, the organic phase was concentrated, and then subjected to column chromatography with the eluent (ethyl acetate / petroleum ether=50 / 1) to obtain 77.6 mg of a light yellow liquid with a yield of 94%.
[0048] 1 HNMR (4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com